Young Stem Cell Vesicles Rejuvenate the Aging Heart: New Study Finds
A new study reveals that tiny vesicles secreted by young stem cells can reverse multiple signs of cardiac aging in mice.
Highlights
- Tiny particles released by young stem cells improved heart function, reduced tissue scarring, and increased stamina in older female mice.
- The treatment reduced inflammation and cellular stress in the heart.
Extracellular vesicles, or EVs, are microscopic particles released by cells that help coordinate activity between tissues. Each vesicle carries molecular cargo, such as proteins and fragments of RNA, that can influence how nearby cells grow, repair damage, or respond to stress. Once dismissed as cellular debris, these vesicles are now being investigated as a core component of how stem cells promote healing.
One subset of EVs, known as small extracellular vesicles (sEVs), has attracted particular interest. These vesicles are commonly produced by adipose-derived stem cells, which are stem cells collected from body fat. While these stem cells have long been studied for their regenerative potential, growing evidence suggests that many of their benefits come from the vesicles they secrete, not the cells themselves.
Now, a new study published in Stem Cell Research & Therapy examines whether sEVs from young adipose stem cells can reverse features of cardiac aging in mice. The researchers found that treatment improved heart structure and function, enhanced physical endurance, and reduced signs of tissue inflammation and damage. These improvements occurred without the need for cell transplants, gene editing, or reprogramming strategies.
Vesicle Therapy Improves Heart Function in Aged Mice
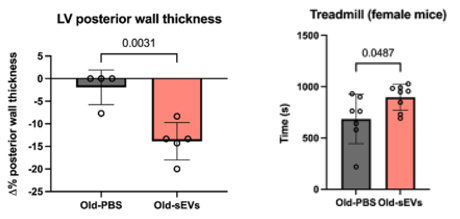
To test the effects of sEVs on aging heart tissue, the researchers treated 22-month-old male and female mice with vesicles collected from the stem cells of young adult mice. The animals received two doses over one week. After a month, their cardiac function was assessed using echocardiography, a non-invasive imaging technique that captures how well the heart contracts and relaxes between beats.
In untreated animals, the scans revealed classic signs of cardiac aging, including thickened ventricular walls and impaired diastolic function – the heart’s ability to relax and fill with blood after each contraction. In contrast, mice treated with sEVs showed thinner heart walls and restored filling capacity, suggesting a measurable improvement in elasticity and performance.
The researchers also evaluated physical endurance using treadmill testing. Notably, treated female mice ran longer before exhaustion than untreated controls, indicating that changes in cardiac function were reflected in whole-body stamina.
Cardiac Tissue Shows Reduced Damage and Inflammation
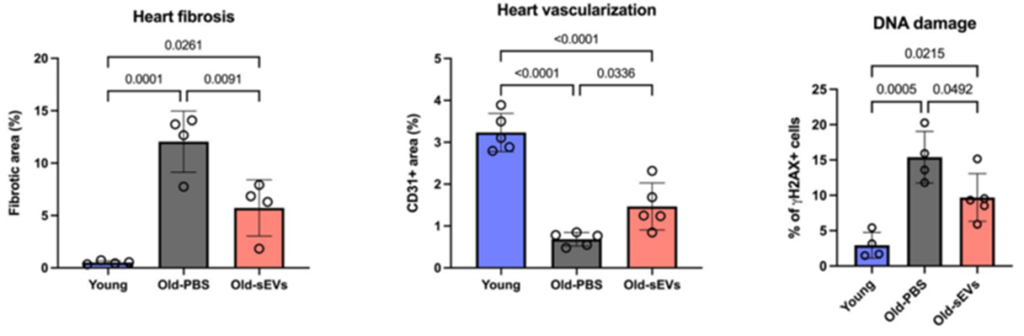
At the cellular level, tissue analysis revealed several key changes. Aged mice typically show higher levels of fibrosis, a form of scarring that stiffens the heart and impairs function. In sEV-treated animals, the amount of fibrotic tissue decreased, and blood vessel density increased. These changes suggest that the vesicles supported both structural repair and vascular remodeling.
Inflammatory markers were also reduced. The researchers measured levels of IL-6 and IL-8, two inflammation-related proteins known to rise with age and contribute to chronic tissue damage. Accordingly, both were significantly lower in treated animals. In addition, markers of oxidative stress were suppressed, indicating reduced molecular damage from reactive oxygen species (ROS) – compounds that trigger oxidative stress.
The study also noted modest reductions in DNA damage and early signs of cellular senescence, which is a state where cells stop dividing and begin secreting harmful molecules. Together, these findings point to broader improvements in tissue environment beyond heart structure alone.
Supporting Function Without Cell Transplants
The therapeutic potential of small extracellular vesicles is not limited to the heart. In aged mice, sEVs derived from young adipose-derived stem cells have been shown to reduce frailty, improve physical performance, and even restore hair regrowth capacity, pointing to a systemic effect on tissue maintenance and repair. These vesicles appear to modulate multiple aging pathways at once, dampening chronic inflammation, reducing oxidative stress, and reactivating cellular programs that become dormant with age.
As a cell-free intervention, sEV therapy offers a practical alternative to stem cell transplantation, sidestepping many of the safety and regulatory concerns associated with introducing live cells into aging tissues. Its compatibility with intravenous (IV) delivery and its ability to cross biological barriers further strengthen its case as a clinically scalable approach.
Although researchers still need to confirm these effects in humans, findings from animal studies suggest that aging can be treated by restoring the body’s ability to maintain and repair its own tissues, rather than attempting to reverse age itself. Instead of functioning as a reset button, small extracellular vesicles appear to work more like a relay system, helping aging cells recover lost communication and regain responsiveness to repair signals. By reactivating these fundamental processes, sEVs offer a promising strategy to preserve tissue health and resilience as the body grows older.
Model: 22-month-old C57BL/6 mice
Dosage: two intravenous injections of 10 micrograms of small extracellular vesicles (sEVs), isolated from young adipose-derived stem cells (ADSCs), spaced one week apart.

