What is Premature Aging and What Can You Do to Prevent or Reverse It
Biological aging is an inevitable process shared by all living organisms. Premature Aging is attributed to chronic health problems and age-related diseases.
Biological aging describes the process of an organism deteriorating cellularly and functionally. About half of human deaths come from age-related diseases like heart disease, diabetes, chronic kidney diseases, chronic obstructive pulmonary disease, stroke, and Alzheimer’s disease. Also, chronic health problems related to the unprecedented aging of the human population in the 21st century threaten to disrupt economies and degrade the quality of later life throughout the developed world.
Premature aging has garnered the attention of scientists all over the globe. This interest stems from the goal of biomedical research to promote the lifespan and healthspan of individuals. Studies of aging disorders, where the cells and organism age at an accelerated rate compared to a normal organism, offer hope to understand the aging process as many of these disorders originate from particular mutations in certain genes.
Causes of Aging
In the past 50 years, scientists have proposed several theories to explain the nature and causes of aging. These theories continue to evolve, fueling a debate of whether aging is programmed in our cells or whether it is a purposeless and unintended accumulation of harmful cellular events that diminish the performance of an organism. Figuring out which of these theories holds the most credulity will require further research, which will provide a better understanding of the mechanisms that induce aging to help scientists mitigate its effects.
Symptoms of Aging
At the cellular level, scientists believe the cause of aging is “cellular senescence,” a state of irreversible growth arrest. Senescent cells accumulate as people age, and they play roles in promoting age-related diseases. Senescent cells result after a certain number of replications, usually 50 to 60 in normal cells.
Causes of cellular senescence include shortening of telomeres, the repeated DNA sequences at the ends of chromosomes that protect the end of the chromosome from deterioration, or fusion with neighboring chromosomes. Telomere shortening occurs with each cellular replication due to incomplete replication of the genome. Other causes include damage to DNA and the epigenome–chemical modifications of DNA and proteins called histones that regulate the activation of genes within the genome.
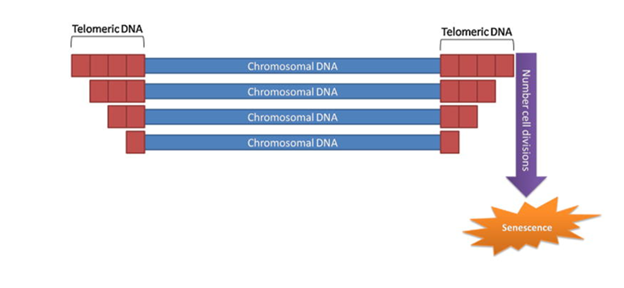
(Pinto da Costa et al., 2016 | Aging Research Reviews) Telomeres decrease in length with each cell division, which can induce senescence.
An accumulation of senescent cells from aging can promote symptoms of inflammation and tissue disruption. Increased senescent cell burden is linked to many age-related diseases, including obesity, diabetes, hypertension, and atherosclerosis. Age-related pathologies can also induce the buildup of senescent cells, which include age-related chronic diseases, cellular stress, hormonal imbalances, chronic infection, certain medications (i.e. chemotherapy or certain HIV medicines), and radiation exposure.
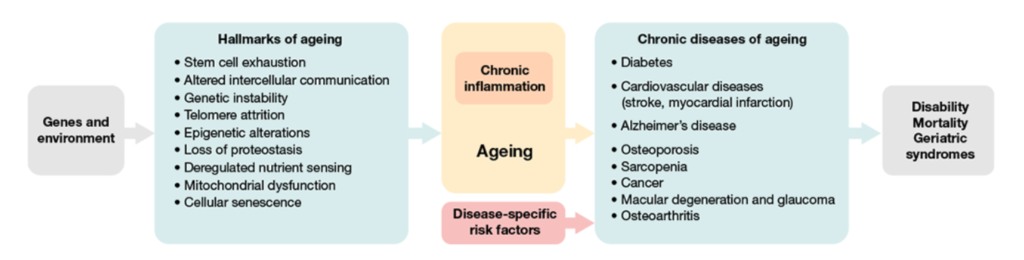
(Campisi et al., 2019 | Nature) Genes and environmental factors contribute to hallmarks of aging that induce chronic inflammation or “inflammaging” that gives rise to chronic diseases of aging.
Diseases that Make You Age Faster
Several diseases lead to premature aging. For example, mutations in DNA can cause premature aging syndromes, including Hutchinson-Gilford progeria syndrome and atypical Werner syndrome. These two conditions are caused by genetic defects in the membrane of the nucleus–the cellular component containing DNA. Other diseases causing premature aging arise from defective DNA repair and DNA maintenance proteins, including classical Werner syndrome, Cockayne syndrome, Bloom syndrome, xeroderma pigmentosum, ataxia telangiectasia, trichothiodystrophy, dyskeratosis congenita, and mosaic variegated aneuploidy syndrome.
Some of the major defects that occur at the level of cells due to these premature aging disorders include genome instability, dysfunctional metabolism, and loss of ability to regenerate tissue. These defects overlap with those that occur during normal aging processes in people. Such overlapping cellular defects suggest common mechanisms behind premature aging syndromes and age-related diseases, making them models for studying normal, physiological aging processes.
Environmental Factors Can Influence How Fast One Ages
Some people age faster than others due to exposure to adverse environmental stressors. Exposure to chemical, biological, and physical agents can reduce the stability and integrity of DNA, leading to DNA replication errors and causing genetic lesions.
Researchers have shown that diet and nutrition are associated with levels of DNA damage and the capacity to repair DNA. For instance, scientists have demonstrated DNA repair capacity increases with higher levels of certain antioxidant agents in the blood.
Telomeres are sensitive to age-related deterioration. Diets, such as the Mediterranean diet, have been shown to slow telomere length shortening in white blood cells (leukocytes), which is linked to anti-inflammatory effects. Slowed telomere length shortening has also been linked with reductions in the occurrence of and death from cardiovascular disease.
Avoiding particular environmental stressors, including excessive sun exposure, a high fat diet, drinking, smoking, stress, or any kind of radiation, can prevent premature aging. Avoiding smoking and drinking can be a way to ensure that your body ages normally. Staying out of the sun for prolonged periods can reduce your chances of DNA damage from UV radiation, which can facilitate aging of the skin. Furthermore, the more you can regulate your stress levels in your life, the more you improve the chances of avoiding premature aging.
How to Prevent Premature Aging
Research has shown that pharmaceuticals can target fundamental aging processes to enhance and extend both health and longevity in experimental animal models, demonstrating that the biological rate of aging can be slowed.
Some examples of interventions that may prevent premature aging with increasing healthspan and lifespan include rapamycin, senolytics, NAD+ precursors, sirtuin-activating compounds, metformin, exercise, and calorie restriction. Rapamycin has shown protective effects against aging in mice and dogs with human clinical trials underway. Senolytic agents target cellular aging and have been shown to have protective effects against age-related diseases in mice. Ongoing human clinical trials for senolytics include studies on arthritis and eye degeneration. NAD+ precursors, including nicotinamide mononucleotide (NMN), are available for human consumption, but few clinical trials have been reported so far. Compounds activating proteins that maintain cellular health, sirtuins, have shown positive results in animal models but have mixed results in humans. Researchers have also associated metformin with an increased lifespan in people with diabetes. Exercise is associated with a reduced risk of age-related disease and improved quality of life through currently unknown processes. Last, calorie restriction, a reduction in food intake, has been shown to enhance lifespan and protect from diseases in worms, flies, mice, rats, and non-human primates and is associated with a decreased probability of disease onset in humans.
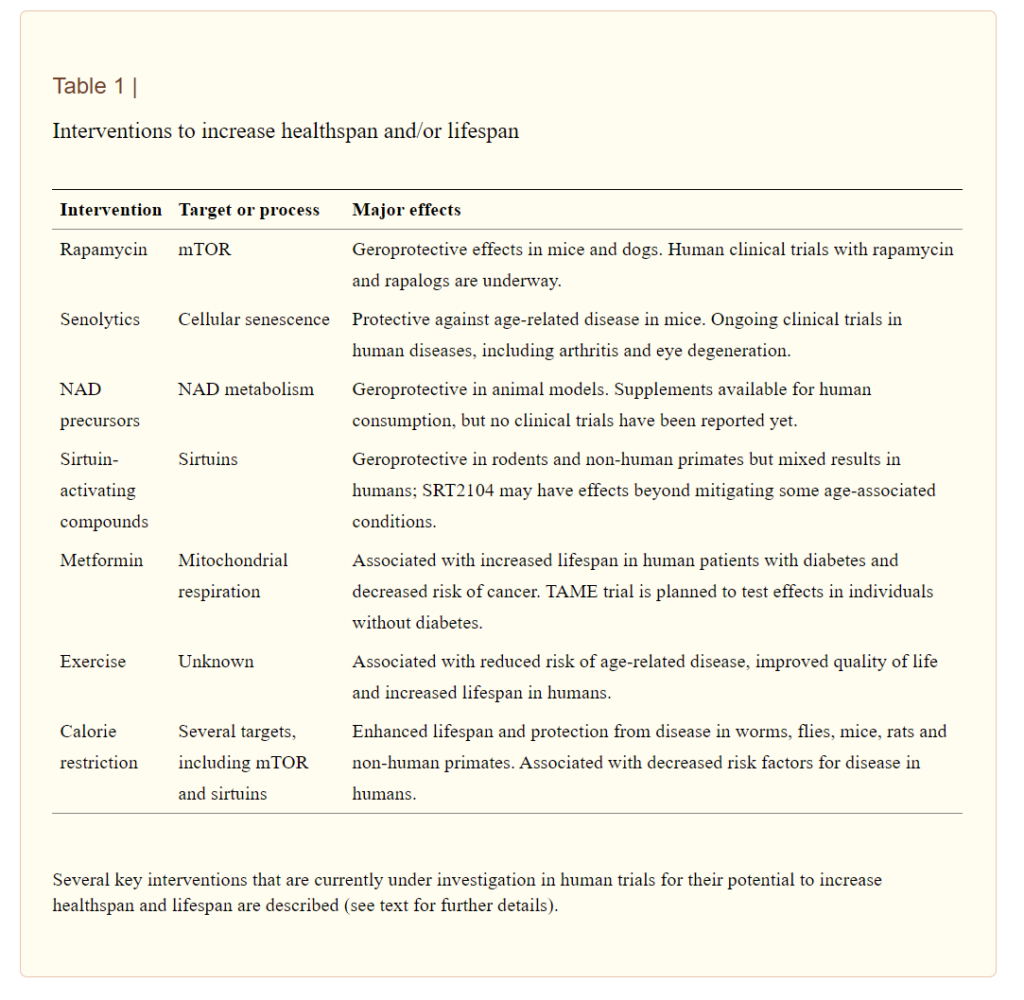
(Campisi et al., 2019 | Nature)
Epigenetic changes may also accompany aging and can speed up related processes. Disturbances in patterns of modifications on DNA called methylation can provoke age-related syndromes, and experimental manipulation of methylation patterns in laboratory animals can modulate lifespan. Perhaps these findings will translate to humans in the future.
Some of these potential therapeutic interventions can improve healthspan and lifespan and thereby prevent premature aging. Clinical trials in humans will reveal the efficacy of these methods. From avoiding environmental stressors like anxiety, depression, or PTSD from childhood trauma, to using pharmacological agents to mitigate the effects of aging, scientists continue their research to find whether we can prevent premature aging.

