What Are Senescent Cells and Why Are They Being Targeted for Healthier Aging?
In recent years, cell senescence has fallen into the biomedical limelight, with these ‘zombie cells’ serving up a rich source of opportunities for innovative therapeutic development in aging and age-related diseases.
Life expectancy has skyrocketed over the last few decades, leaving a billowing trail of diseases linked to aging. An ever-increasing number of scientific studies have honed in on cell senescence as a major contributor to the development of these age-related disorders.
For these reasons, cell senescence has become a focus for research institutions and biotech companies that target it to ameliorate a variety of human conditions. There are even new classes of drugs being developed that are meant to specifically eliminate senescent cells from the human body.
So, what exactly is cell senescence, what triggers cell senescence, and what do we know about how to get rid of senescent cells?
What is the definition of cell senescence?
Back In 1961, a couple of researchers by the names of Hayflick and Moorhead tried out an experiment to see if human cells could be replicated indefinitely in a lab dish. What they found was that these cultured human cells do not replicate forever but start to slow their divisions and enter an irreversible dormancy. This state of arrest in cell division is called cell senescence (the term senescence derives from the Latin “Senex”, which means “old man” in Latin).
But these cells don’t die off. In fact, they have an increased resistance to cell death by upregulating pathways critical to cell survival. They also begin to do some weird things, like change their shape and size as well as secreting inflammatory molecules, which, in turn, can cause other cells to become senescent.
What causes cell senescence?
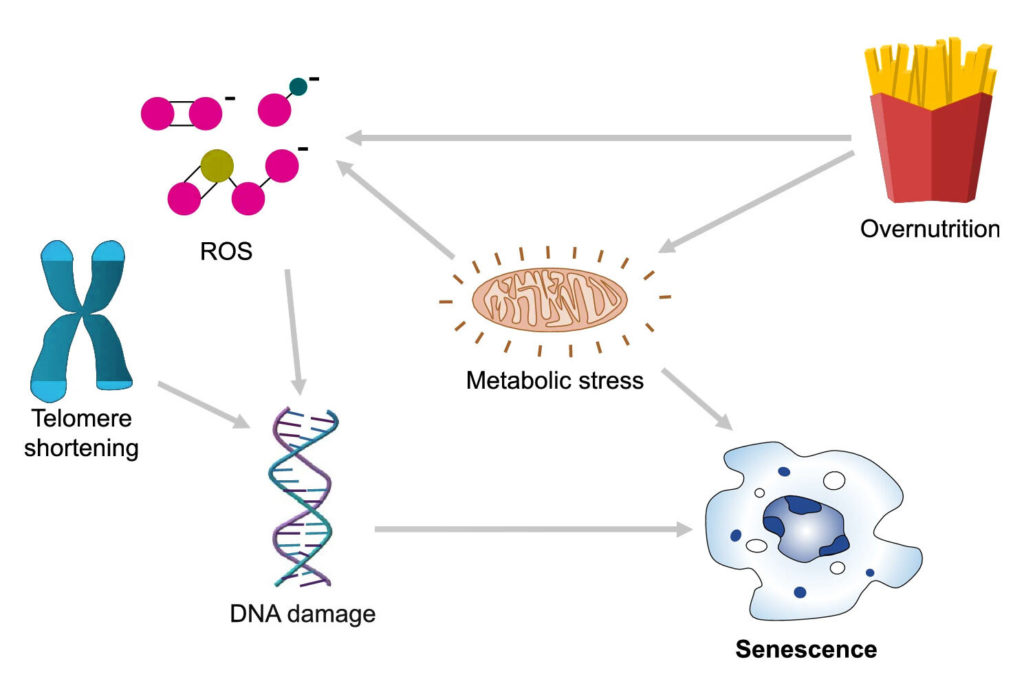
Senescence seems to be triggered when cells hit a critical point where they no longer can take on more damage or stress. This often is connected to the integrity and maintenance of each cell’s genetic blueprint, or DNA.
Cell damage and stress can have external sources, like toxins, viruses, and radiation. For example, when we catch some sun, the UV rays can cause DNA damage, which puts cells in an alerted frenzy to repair and maintain the integrity of our genetic code. There are more sources of damaging radiation, such as X-rays, which is why medical and dental technicians give you a lead shield to protect your cells and DNA from getting zapped.
Other kinds of sources for cell damage and stress are internal — self-generated, naturally occurring from basic, everyday cellular processes. For example, as a byproduct of metabolism, which is how our cells convert food into energy, cells generate DNA damaging molecules called reactive oxygen species (ROS). When there is an abundance of ROS and our cells are unable to detoxify these damaging products with antioxidants, cells enter a state that’s called oxidative stress in which our DNA is highly susceptible to DNA damage.
Another source of internal stress relates to the experiments performed by Hayflick and Moorhead on a limit to cell replication. Our chromosomes — the long strands of DNA that bunch up to look like X’s — have protective caps of repetitive DNA called telomeres that with every cell replication get trimmed. But these caps are only so long and can only protect from so many cell replications. So, when these cushions are whittled away to the point where the DNA coding our genetic blueprint is exposed, cells enter what’s called telomeric senescence and stop replicating.
Senescence also appears to be closely linked to how much and what we eat as overnutrition may also lead to cell senescence. Fat tissue from obese mice seems to be littered with senescent cells. Also, obese individuals display increased production of ROS in fat tissue.
Senescent cells and aging
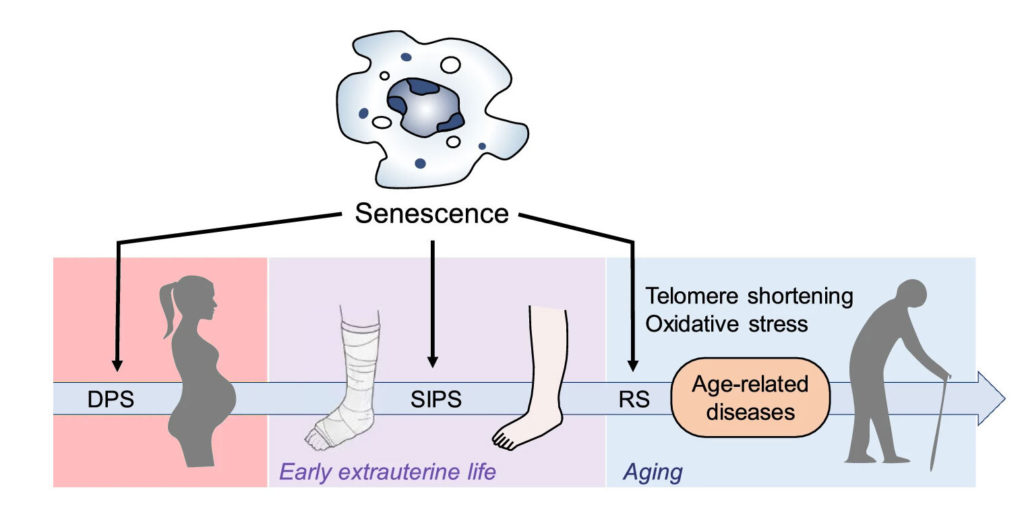
The link between aging and senescence has been well established. Simply put, as we age, our cells continue to be exposed to stress, which, ultimately, leads to an increase in the number of cells that become senescent. But do senescent cells actually affect aging?
There have been several recent experiments where researchers depleted senescent cells in aging animals, which postponed the onset of age-related diseases and extended lifespan. These kinds of studies have ignited clinical and research interest and inspired the development of targeted drugs to eliminate senescent cells associated with age and disease.
What is the purpose of cell senescence?
Scientists think that one of the main reasons that our cells have gained the ability to engage in this program that causes replicative arrest is that senescence prevents the replication of cells harboring damaged DNA. This serves a critical function in preventing cancer and limiting tissue damage by stopping the propagation of faulty cells.
Senescence plays a critical role during our development. Senescent cells have been identified in the developing embryos of multiple species including humans, birds, amphibians, and fish. This indicates that the role of senescence in animal development is widespread. The role of senescent cells appears to be in the fine-tuning of organ development, where superfluous cells become senescent and then get targeted and eliminated by the immune system.
How can we fight cellular senescence?
Strategies against cell senescence that can be translated into therapies for use in humans may be classified into the following groups:
- non-pharmacological interventions that prevent the accumulation of senescent cells
- pharmacological therapies aimed at reducing the amount of pro-inflammatory molecules secreted by already existing senescent cells
- pharmacological therapies aimed at reducing the number of senescent cells in the organism (or what researchers call senolytics)
As it pertains to non-pharmacological interventions, some studies have shown that calorically restricted diets started in adulthood can reduce the number of senescent cells in proliferating tissues. Although not fully understood, calorie restriction is widely recognized to extend longevity and in mice reduces senescent cell burden.
Over the last few years, the depletion of senescent cells with pharmacological therapies in animal models has demonstrated striking results across multiple tissues, organs, and at the whole animal level and this continues to inspire the development and refinement of therapy. However, as yet, their efficacy and safety have not been extensively tested in humans. The successful development of therapies that target senescent cells for clinical use will allow us to treat multiple age-related conditions like cancer, atherosclerosis, diabetes, immune dysfunction, neurodegeneration, neurovascular events, pulmonary fibrosis, osteoporosis, and impaired vision.
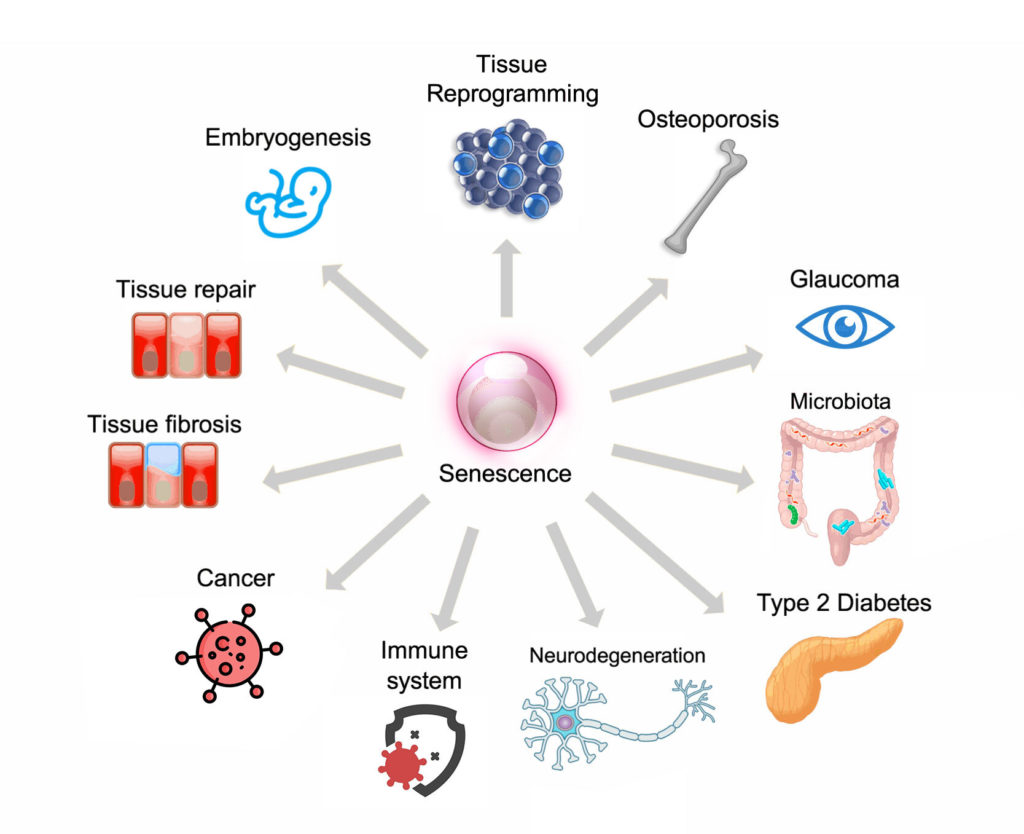
Chronic non-communicable diseases are strongly associated with aging and constitute a top health priority for every country in the world. An ushering in of this new era of therapeutics that target senescent cells offer the appealing prospect of simultaneously targeting the shared biological processes that underlie many of these diseases as well as extending lifespan and healthspan.
As it stands, research on cell senescence is nowhere near arresting, but, rather, continues to grow at an accelerated pace.

