Washington University Study Shows NMN Replenishes NAD+ in the Aging Brain
Scientists show that treating aged mice with nicotinamide mononucleotide (NMN) or small vesicles containing the NAD+ synthesizing enzyme NAMPT (eNampt) increases NAD+ levels in regions of the hypothalamus - a brain region that regulates homeostasis.
Highlights:
- Scientists have developed a novel method to accurately quantify NAD+ levels in the brain, specifically in small tissues.
- Supplementing aged mice with NMN restores NAD+ levels in three of four hypothalamic regions.
- Injecting aged mice with small vesicles containing an NMN-synthesizing enzyme – NAMPT (eNAMPT) – raises NAD+ levels in four subregions of the hypothalamus.
Our brain is a highly sophisticated organ composed of many harmonious networks, with each subregion holding a unique function that helps sustain overall health and survival. Scientists have pinpointed NAD+ as a critical molecule that helps preserve brain health. However, NAD+ declines with aging, and its depletion, particularly in the hippocampus – the brain region responsible for memory – has been linked to cognitive deficits. That being said, until now, little has been known about whether NAD+ declines in other vital brain regions like the hypothalamus.
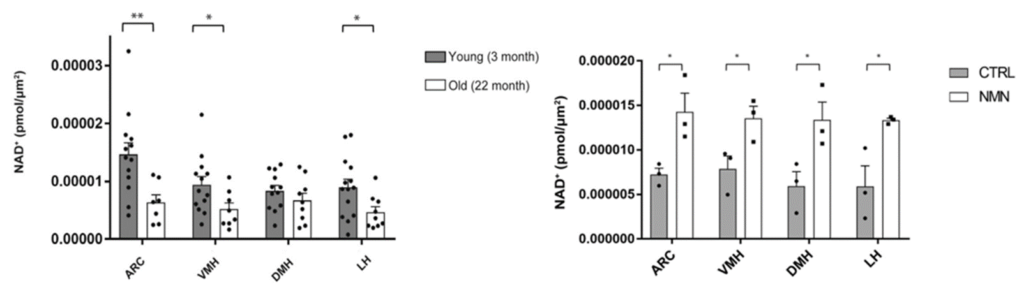
In a new study published in the journal NPJ Aging, researchers from Washington University employ a newly-established method to accurately measure hypothalamic NAD+ levels in young and aged mice. Imai and colleagues show NAD+ levels fall significantly in three of the four measured subregions in the hypothalamus. Accordingly, injecting 22-month-old aged mice with one shot of 300 mg/kg NMN restores NAD+ levels in all three regions. Furthermore, the investigators find that injecting aged mice with small vesicles from young mice containing the NAD+ synthesizing enzyme NAMPT boosts NAD+ levels by nearly 50% in the hypothalamus.
NMN Restores Declining NAD+ Levels in the Hypothalamus
Prior to this study, scientists lacked the technology to measure NAD+ levels in small tissues accurately. Given that the hypothalamus is split into extremely small subregions called nuclei, minimal data exists surrounding NAD+’s role in hypothalamic aging. Using a new combinatorial methodology of NAD+ measurement, Imai and colleagues successfully showed that aged mice exhibited declining NAD+ levels in three of the four measured subregions of the hypothalamus. Moreover, the initial findings confirm that NAD+ levels decrease in multiple brain regions, opening the door for future studies looking at the effects of reduced NAD+ in the hypothalamus.
Given that NMN is a potent NAD+ replenisher in many tissues, the investigators examined whether injecting aged mice with NMN could rescue the fallen hypothalamic NAD+ levels. Thirty minutes after the initial injection, aged mice displayed increased NAD+ levels in all four measured subregions of the hypothalamus. Moreover, the findings demonstrate that NMN attenuates declining NAD+ levels in the hypothalamus of aged mice.
NAMPT-Containing Vesicles Boost Hypothalamic NAD+ Levels
NAMPT is a well-established NAD+ enhancer in multiple tissues and a longevity promotor, with previous research showing that transplanting small vesicles from young mice raises NAD+ and prolongs lifespan in aged mice. With this in mind, Imai and colleagues explored whether transplanting similar vesicles could exert NAD+-boosting effects in the hypothalamus.
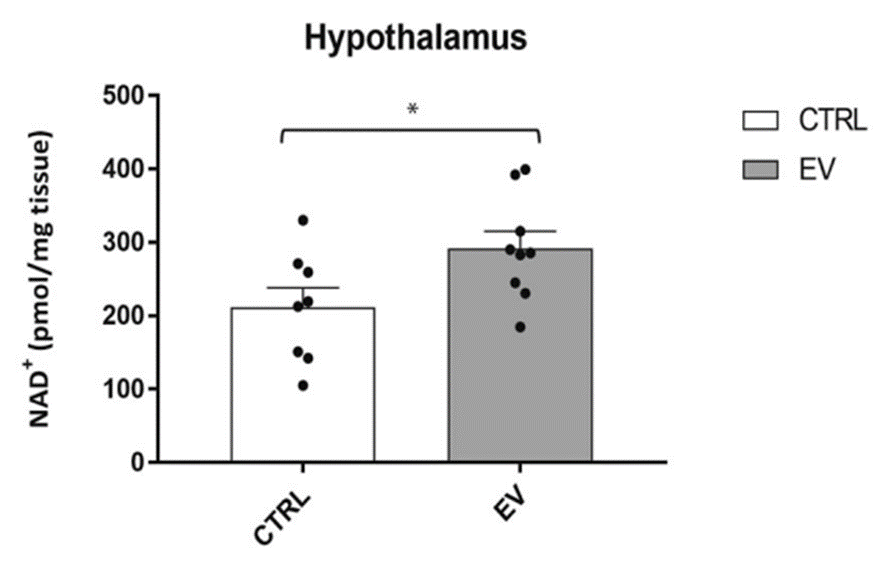
The findings showed that injecting aged mice with NAMPT-containing vesicles from young mice leads to significantly higher levels of NAD+ in the hypothalamus. Given that the findings demonstrate that these vesicles hold potent NAD+-boosting effects in the hypothalamus, it would be interesting to see if this treatment could alleviate the age-related hypothalamic changes in sensory processing and emotional control.
NMN and eNAMPT as Potential Therapeutic Interventions for Brain Aging
With Imai and colleagues establishing a new mode of accurately quantifying hypothalamic NAD+ levels, we can better monitor hypothalamic aging and potentially identify novel therapeutics. Our brains inevitably experience an age-associated functional decline due to continual harm from stressors like inflammation and DNA damage, both of which rely on NAD+ for attenuation. Thus, finding ways to replenish NAD+ in multiple tissues is vital to curbing aging and organ deterioration. The current findings demonstrate that NMN and eNAMPT successfully restore NAD+ levels in the aged hypothalamus, highlighting that these treatments could improve aspects of brain aging, like poorer sleep quality, decreased physical function, and impaired cognition. However, more comprehensive studies are needed to confirm these hypotheses.
Model: C57BL/6J mice
Dosage: 300 mg/kg NMN intraperitoneal injection at 22 months of age

