Stem Cell Secreted Vesicles Counteract Aging: Latest Study
Researchers show that stem cell vesicles (extracellular vesicles [EVs]) reduce cellular senescence — where cells enter a state of non-proliferation and release inflammatory molecules — largely due to snippets of RNA within the vesicles.
Highlights
- EV treatment reduces cellular senescence and restores cellular proliferative capabilities.
- Two short snippets of RNA called microRNAs (miRNAs) within the extracellular vesicles — miR-15b-5p and miR-290a-5p — rejuvenate aged cells when transfected into them.
- Treating mice with stem cell-derived EVs reduces levels of proteins linked to senescence in multiple organs, including the liver, kidney, and spleen.
Extracellular vesicles (EVs) are tiny, membrane-bound sacs containing various molecular cargo, including proteins, DNA, and/or RNA that cells secrete as a means of communication. According to research, treatment with EVs derived from embryonic stem cells recapitulates many of the rejuvenation effects of stem cell therapy. Since stem cell therapies are limited by immune rejection and tumor growth promotion, not to mention ethical issues, resolving whether EVs confer similar benefits as embryonic stem cells without unwanted effects is paramount. Moreover, identifying which EV components rejuvenate cells may lead to better anti-aging therapies to replace stem cells.
Published in Bioactive Materials, Liu and colleagues from Nankai University in China show that treating senescent cells with embryonic stem cell derived EVs mitigates their senescence. The researchers went on to identify two RNA snippets — miR-15b-5p and miR-290a-5p — that were elevated in the EVs, which participate in longevity-related pathways, and that were found to rejuvenate aged cells. Liu and colleagues then showed that treating aged mice with EVs lowered levels of protein markers of senescence in multiple organs such as the liver, kidney, and spleen. These findings confirm the method of culturing stem cells and using their EVs to counter aging without the ethical concerns and adverse side effects that go with embryonic stem cell therapies. They also illuminate elements within stem cell derived vesicles that confer anti-aging benefits, opening the possibility of applying them to new therapies against aging.
Embryonic Stem Cell Extracellular Vesicles Ameliorate Cellular Senescence and Rejuvenate Multiple Tissues
Liu and colleagues sought to confirm that embryonic stem cell derived extracellular vesicles mitigate cellular senescence in cells. Senescence plays a powerful role in aging since senescent cells accumulate during aging and secrete inflammatory factors to other surrounding cells, initiating their senescence along with systemic inflammation.
Along those lines, since cells become senescent after a certain number of divisions, Liu and colleagues used mouse cells that went through seven divisions, leading to their senescence. Treating these senescent mouse cells with EVs alleviated several protein markers of cellular senescence and reversed the senescence-typical cell structural characteristic of being flat. Extracellular vesicle treatments also enhanced the activation of genes tied to cell proliferation to levels comparable to young, healthy cells. These results show that EV treatment rejuvenated senescent mouse cells.
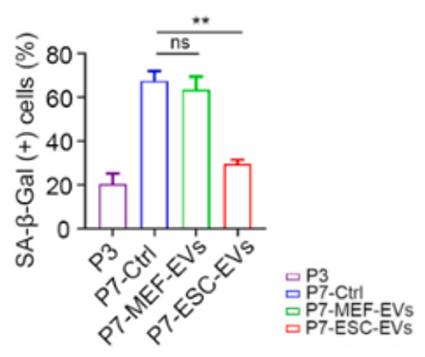
Since Liu and colleagues had established that EVs rejuvenate senescent cells, the big question became what cargo within the EVs was sufficient and necessary for cellular rejuvenation. Previous research had shown that EVs contain miRNAs — short RNA snippets that regulate protein expression — some of which have been shown to slow aging. Hence, the China-based researchers focused their attention on miRNAs within embryonic stem cell EVs. They found that compared to normal EVs — EVs from non-stem cells — embryonic stem cell EVs exhibited 128 miRNAs with elevated levels. Many of these miRNAs were found to play roles in longevity-related pathways like mTOR and insulin signaling.
The researchers narrowed their list down to six miRNAs that showed high levels in embryonic stem cell EVs and low levels in aged cells. When the researchers transfected aged mouse cells with synthetic copies of the six miRNAs that mimic miRNA function, they identified two miRNAs that were especially effective at alleviating cellular senescence — miR-15b-5p and miR-290a-5p. These data suggest that miR-15b-5p and miR-290a-5p miRNAs are sufficient and necessary molecular cargo within embryonic stem cell EVs for cellular rejuvenation.
To find whether embryonic stem cell EVs rejuvenate not only cells in a dish but also entire organisms, Liu and colleagues tested EV injections on aged mice. They found that EV treatment shifted aged mouse gene activity profiles (the transcriptome) closer to more youthful ones. Furthermore, embryonic stem cell EV treatment reduced the abundance of senescence-related proteins in organs like the liver, kidney, and spleen. These findings support that embryonic stem cell EV treatment can also rejuvenate live animals.
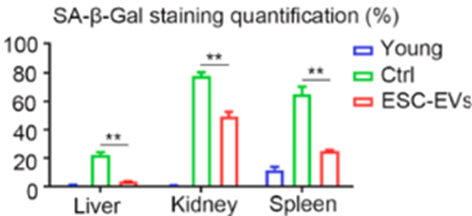
“We evaluate the anti-senescence effects of [embryonic stem cell EVs] in vivo, suggesting a new non-cellular therapeutic tool for aging and age-related diseases,” said Liu and colleagues.
Future Prospects of Using Stem Cell Vesicle Therapies Against Aging
The study showed that EVs from embryonic stem cells effectively mitigate senescence and restore more youthful gene activity and senescence-related protein levels. The study also provides an indication that two RNA snippets, miR-15b-5p and miR-290a-5p, are sufficient and necessary for embryonic stem cell EVs’ anti-aging benefits.
The study isn’t the first to confirm the benefits of embryonic stem cell EVs in a live animal model. Previous experiments showed that embryonic stem cell derived EVs improve kidney function in a mouse model of kidney injury. The study is, however, the first to show that embryonic stem cell EVs reduce protein markers of senescence in multiple mouse tissues.
If applicable to humans, embryonic stem cell EVs could be a new way to rejuvenate organ health and may apply more broadly as an overall anti-aging therapy. Future research needs to uncover what EV doses are most effective and for what lengths of time EV treatments are necessary to invoke positive benefits against aging.
Model: Embryonic fibroblast cells from C57BL/6 mice; C57BL/6 mice
Dosage: 100 µg of embryonic stem cell extracellular vesicles injected once every two days for eight weeks

