Top NMN Supplement Delivery Systems
Capsules are the most researched NMN delivery system in humans, but mouse studies suggest that the powdered form of NMN could have similar benefits.
Highlights:
- There are many different methods for delivering NMN on the market, including capsules, powder, suppositories, mouth sprays, and nasal sprays.
- The top delivery system may be capsules that are pH-sensitive and designed to bypass the acidity of the stomach.
Individuals contemplating taking NMN supplements may be wondering about its best mode of delivery. While many overlook this detail, NMN’s route into the body can ultimately determine its benefits to the user. An appropriate delivery system will ensure maximum bioavailability — the fraction of ingested compound that reaches the bloodstream in its active form. And the effectiveness of a supplement greatly depends on this bioavailability.
Considering that delivery systems work differently with each compound, most NMN delivery systems are not backed by clinical research. Thus, capsules are the only NMN delivery system that has been tested repeatedly in humans. However, this does not necessarily mean that capsules are the top delivery system. Therefore, each of the most common NMN delivery systems currently on the market will be discussed in terms of their potential bioavailability in humans.
NMN Capsules

Human studies almost exclusively utilize encapsulated NMN supplements. In many of these studies, NMN has been shown to increase blood NAD+ levels, suggesting NMN’s robust bioavailability. Furthermore, one study has shown that while 600 mg of encapsulated NMN raises blood NAD+ levels, 900 mg does not increase NAD+ levels further. Such a plateau in blood NAD+ levels within this dosage range suggests there may be a limit to how much you can increase your NAD+ levels with this delivery system.
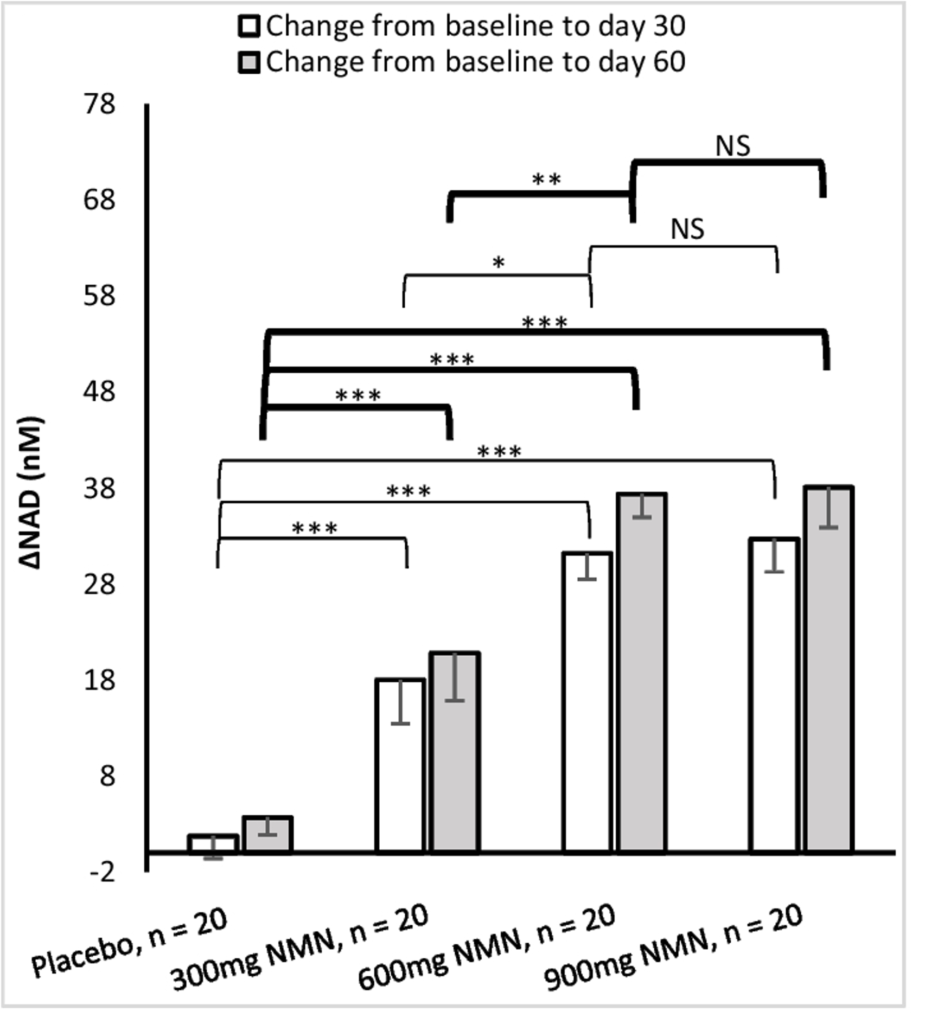
(Yi et al., 2022 | GeroScience) Dose-Dependent Changes in Blood NAD+ Levels. After 30 (white) and 60 (gray) days, 300 mg of NMN increases NAD+ levels (ΔNAD) compared to placebo. A 600 mg dose increases NAD+ levels more than 300 mg, but 900 mg does not increase NAD+ levels further.
In some cases, special capsules called enteric capsules can enhance the bioavailability of compounds by exploiting the varying pH levels of our gastrointestinal (GI) tract. The pH of our stomach is very acidic, making it capable of destabilizing many compounds. However, pH-sensitive enteric capsules can protect their contents by bypassing destruction in the stomach and dissolving in the intestines. In this way, enteric capsules could increase NMN’s bioavailability, but this has not been tested extensively.
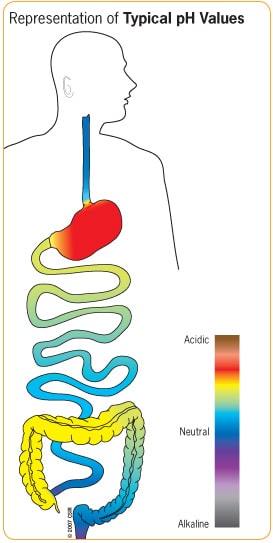
(GI Society) Typical pH Levels of Gastrointestinal (GI) Tract. While the stomach is very acidic (red), and much of the small and large intestines are neutral (blue), the colon has pH values that lie in between (yellow), allowing for targeted delivery with pH-sensitive enteric capsules.
NMN Powder
Powdered compounds are often placed into capsules, which have the advantage of concealing unpleasant tastes and preventing absorption in the mouth. However, some individuals may prefer to dissolve powdered supplements into water to avoid having to swallow pills. This may be a feasible option for NMN considering that NMN remains stable when dissolved in water.
Furthermore, many studies have shown that when NMN is placed into the drinking water of mice, it raises their blood NAD+ levels, suggesting its bioavailability. Additionally, one study has shown that NMN doesn’t significantly degrade in simulated stomach acid, which has a similar pH to our stomach acid. Therefore, enteric capsules may be unnecessary when it comes to the bioavailability of NMN. More human studies will be needed to determine if the capsule form of NMN is more bioavailable than the powdered form dissolved in water.
NMN Suppositories
NMN suppositories are similar to NMN capsules, whereby NMN powder is placed into a durable material. However, suppositories are larger and can store more NMN. Suppositories also bypass the stomach, so their contents may be less prone to degradation. However, whether the NMN suppository delivery system makes NMN more bioavailable than encapsulated NMN has not been tested. Additionally, many individuals may find suppositories uncomfortable and difficult to administer on a regular basis.
NMN Mouth Spray and Sublingual NMN
With NMN mouth spray, NMN is meant to be absorbed through the mouth, which bypasses the GI tract and allows for rapid absorption into the bloodstream. In addition, oral sprays typically contain liposomal NMN rather than the usual microcrystalline NMN. Liposomes are bubble-like structures made of natural fats that mimic the structures made by our body to transport various molecules. For this reason, liposomal NMN is thought to be more bioavailable than crystalline NMN.
Other NMN delivery systems similar to mouth sprays include sublingual gels, tablets, and powders. These delivery systems may sustain the absorption of NMN longer than sprays as the NMN may remain in the mouth for longer periods. Furthermore, the floor of the mouth below the tongue may be more permeable to NMN than the rest of the mouth, making it more bioavailable. However, whether NMN absorbed via the mouth has similar bioavailability to NMN absorbed through the GI tract is unknown.
Nasal Spray
The nasal route of administration for compounds has the advantage of bypassing both the GI tract and blood-brain barrier (BBB). The BBB stops potentially harmful molecules in the peripheral bloodstream from getting to the brain. As a consequence, supplements and drugs that cannot cross the BBB cannot reach the brain when orally administered. However, mouse studies have shown that NMN crosses the BBB, so nasal administration may be unnecessary. Additionally, the nasal cavity can degrade compounds and it is unclear how effectively NMN nasal spray raises NAD+ levels in the brain or the bloodstream.
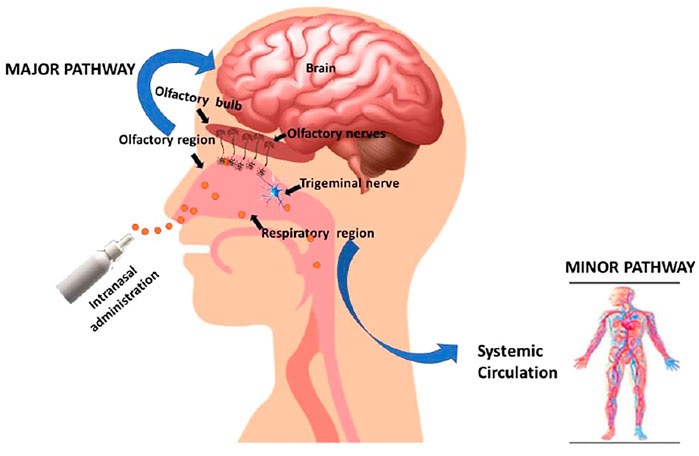
(Pandey et al., 2022 | Front. Pharmacol.) Nasal Delivery System. Compounds administered intranasally can be directly absorbed by neurons of a nerve in the nasal cavity called the trigeminal nerve. However, this route does not substantially increase levels of the compound in the rest of the body (systemic circulation).
Topical NMN for Skin
The topical application of NMN involves NMN being dissolved into oily solutions to be rubbed onto the skin. A study has shown that topical NMN reduces inflammation and skin damage in a mouse model for eczema. Another study has shown that NMN raises NAD+ levels in skin cells grown in a lab dish. Therefore, it may be possible that topical NMN raises NAD+ levels in the skin of humans. However, topical NMN may not substantially raise blood NAD+ levels. Furthermore, raising blood NAD+ levels with other delivery systems could also raise NAD+ in the skin.
The Top NMN Delivery System
Because most human research has only been done on microcrystalline encapsulated NMN supplements, determining if other delivery systems are comparable must be primarily based on animal studies. At the same time, a Japanese study has shown that injecting NMN into healthy individuals raises blood NAD+ levels by 20% and lowers blood fat levels. Since injectables bypass all the body’s natural barriers, injecting NMN directly into the bloodstream makes it virtually 100% bioavailable. The caveats with injectable NMN are the invasiveness and safety concerns involved with puncturing the skin with a needle. Additionally, there is a lack of injectable NMN products on the market.
In most rodent studies showing NMN supplementation increases blood NAD+ levels, the researchers either injected NMN, dissolved NMN into drinking water, mixed NMN into food, or force-fed (oral gavage) NMN to the rodents. Aside from injections, these administration methods are similar to drinking NMN dissolved in water or another liquid. Thus, powdered forms of NMN dissolved in liquid or foods may have similar effects to NMN capsules. Still, since enteric capsules may enhance bioavailability, they could be considered the top NMN delivery system. More research comparing NMN delivery systems will make this more clear.

