New Study Shows Senomorphic Flavonoids Boost Cancer Treatment Efficacy
Researchers find that the flavonoid rutin is a potent senomorphic that enhances chemotherapy's ability to reduce tumor size in mice.
Highlights:
- In vitro, rutin successfully decreases the activity of the senescence-associated secretory phenotype (SASP) – compounds secreted by senescent cells that drive inflammation and support tumor growth.
- Combining rutin with the chemotherapeutic drug MIT is more successful at reducing tumor size than treatment with MIT alone.
The older we get, the more senescent (aged) cells accumulate throughout the body, slowly triggering the development of several age-related diseases like cancer. This happens, in part, due to the senescence-associated secretory phenotype (SASP). Notably, some evidence suggests that senescent cells and SASP factors make cancer cells more resistant to treatments like chemotherapy. Because of this, scientists are exploring whether senomorphics – compounds that target SASP factors – can mitigate this resistance and enhance the efficacy of chemotherapeutics.
As reported in the journal Aging Cell, researchers from Binzhou Medical University in China examined the senomorphic potential of the natural flavonoid rutin and its ability to curtail chemoresistance. Lui and colleagues confirmed that rutin exerts senomorphic effects, reducing the activity of several SASP factors in senescent human prostate stromal (PSC27) cells. In vitro analysis of prostate cancer cells grown in media with senescent PSC27 cells revealed that cancer cell viability is decreased with the combined treatment of rutin and the chemotherapy drug MIT. Furthermore, the investigators found that rutin increases the potency of MIT in mice, leading to significantly smaller tumors.
Rutin Mitigates the SASP
While it is true that senescent cells promote cellular toxicity and drive age-related diseases, some senescent cells play vital roles in key physiological processes like wound healing, tissue repair, and embryonic development. Because of this, aging scientists have been tasked with developing senolytic compounds that can strictly target harmful senescent cells without completely eradicating the useful ones. However, some researchers have shifted gears and turned to senomorphic compounds, which target the pro-inflammatory SASP factors rather than senescent cells themselves. This unique approach not only circumvents the challenge with senolytics but also helps suppress the tumor-promoting environment tied to SASP molecules, highlighting the potential of utilizing senomorphics in cancer therapeutics.
Lui and colleagues sought to determine if rutin could blunt the SASP without affecting cellular senescence. To do this, the investigators treated senescent PSC27 cells with rutin and examined the activity of multiple SASP factors. The results showed that rutin-treated cells exhibited significantly lower SASP activity than untreated cells. However, both groups displayed similar concentrations of senescent cells, demonstrating that rutin targets the SASP without affecting cellular senescence. Moreover, these initial findings indicate that rutin is an effective senomorphic agent.
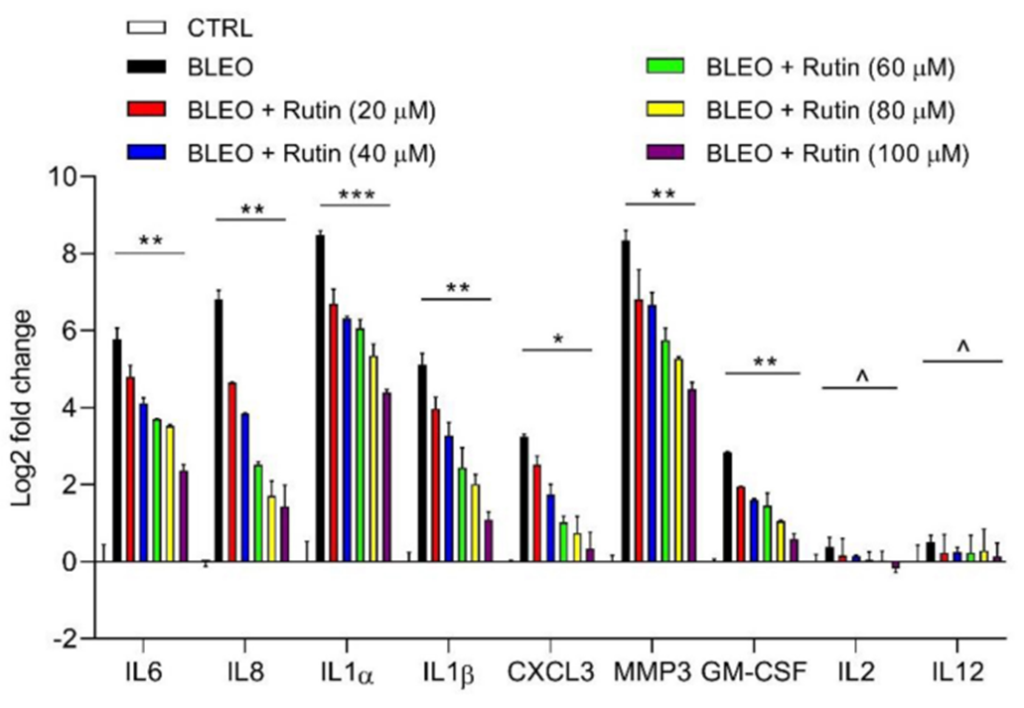
Rutin Enhances Chemotherapeutic Efficacy
Chemotherapy is an established cancer treatment that can successfully extinguish cancerous cells. However, studies suggest that, upon treatment, some cancer cells transition to a senescent state, forming new senescent cell populations and pro-inflammatory SASP factors that further exacerbate cancer progression and tumor growth. Notably, scientists have shown that these re-emerged cancer cells are more resistant to chemotherapeutic treatment, which significantly reduces the chances of remission.
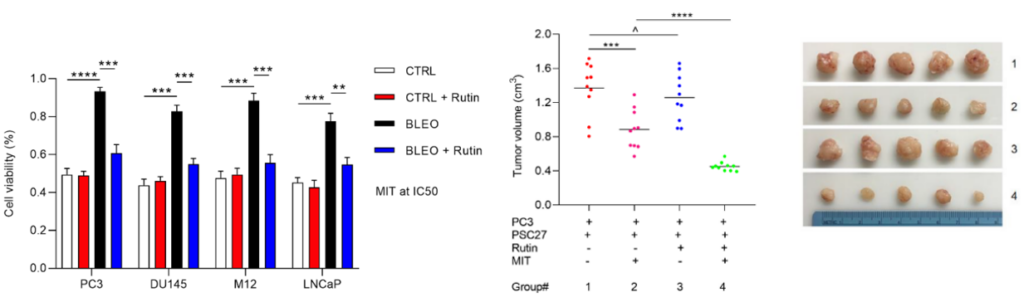
Lui and colleagues posited that rutin’s potent senomorphic properties could reduce the chemoresistance of cancerous cells and enhance chemotherapeutic efficacy. To explore this matter, the investigators grew prostate cancer cells in media with senescent PSC27 cells and examined whether the combined treatment with rutin and the chemotherapeutic MIT could decrease cancer cell viability. Accordingly, the results showed that cancer cell viability was high in the dish treated with just MIT, demonstrating chemoresistance. Conversely, cancer cell viability was drastically diminished in cells treated with both rutin and MIT, indicating that rutin suppresses chemoresistance and boosts chemotherapeutic efficacy.
Due to the positive results in vitro, the investigators wanted to see if combining rutin and MIT could attenuate tumor growth in mice. To induce tumor growth, Lui and colleagues implanted modified tissue (tissue recombinants) containing prostate cancer cells and senescent PSC27 cells into the hind flank of experimental mice. Then, mice were treated with either rutin, MIT, or both rutin and MIT.
Compared to untreated mice, those treated with MIT alone displayed tumors with much lower volumes, demonstrating that MIT is an effective chemotherapeutic. However, combining MIT with rutin led to even smaller tumor volumes in mice, further indicating that rutin’s senomorphic properties potentially amplify the efficacy of chemotherapy and reduce chemoresistance.
Rutin’s Potential Ties to Longevity
Flavonoids – plant-based polyphenols – have become a research hotspot in the field of aging, as they hold powerful antioxidative and anti-inflammatory properties. Rutin is part of the flavonoid family and is structurally comprised of the carbohydrate rutinose and the polyphenol quercetin, another compound with well-established health benefits, particularly in aging muscles. Research over the past decade has demonstrated rutin’s potential to prolong longevity, with studies showing it exerts neuroprotective, anti-diabetic, anti-tumor, and cardioprotective effects. Interestingly, one study found that an altered form of rutin (sodium rutin) can successfully increase the lifespan of mice by 10%. Collectively, it appears that rutin holds great potential as a longevity intervention, but more human studies are needed to fully elucidate its pharmacological effects.
Model: Human prostate stromal cells (PSC27)
Mice implanted with tissue recombinants containing PSC27 sublines and prostate cancer (PC3) cells
Dosage: In vitro: 100 uM Rutin
In vivo: 10 mg/kg rutin on 1st day of week 3, 5, and 7 after tumor implantation

