The Combination of Melatonin and NMN Protects the Aged Heart from Injury
This treatment is a promising strategy to attenuate damage and boost protection in aged hearts
Highlights
- In aged rats with myocardial injury, NMN pre-treatment and melatonin post-treatment had significant cardioprotective effects.
- The combination of NMN and melatonin can be a promising strategy to attenuate myocardial damage in aged hearts.
One major cause of heart failure is what is known in the medical field as myocardial ischemia/reperfusion (I/R) injury — heart damage that occurs when blood flow to the heart stops (ischemia) and then restarts (reperfusion). We also now know that aging increases our susceptibility to myocardial ischemia and limits the recovery of heart function after the ischemic insults. On top of that, what is most troublesome is that aged ischemic hearts are resistant to protection by routine therapeutic strategies.
Research from Hosseini and colleagues published in the Journal of Cardiovascular Pharmacology and Therapeutics revealed the therapeutic potentials of nicotinamide mononucleotide (NMN) and melatonin following myocardial I/R injury in aged rats, specifically when administered in combination. NMN and melatonin had protective effects against I/R injury in aged rats by decreasing the injury size and improving myocardial function as well as other measures of heart cell damage and dysfunction. This shows that the combination of melatonin and NMN can be a promising strategy to attenuate myocardial I/R damage in aged hearts.
Treating Damage from the Stop and Go of Blood Flow to the Heart
During the restoration of blood flow, the heart is affected by inflammation as well as the overproduction of harmful oxygen containing-molecules called reactive oxygen species (ROS), dysfunction of the cell’s power-generating structure (mitochondria), and death of heart cells. These effects are mirrored in heart aging, which results in increased dysfunctional mitochondria, overproduction of reactive oxygen species (ROS), and decreased ability of the heart to contract. So, these aspects of heart disease and aging are key targets for treatment.
However, most preclinical studies — the ones that precede human studies — are usually performed using young animal models and focus on just one treatment and very limited aspects of the disease in question. This doesn’t really recapitulate human patients with myocardial I/R injuries, who are generally older and exhibit other comorbidities or heart disease risk factors. So, cardioprotective treatments that come through experimental pipelines using young animal models do not translate to humans well, usually failing to reduce infarct size and improve patient outcomes in clinical settings of myocardial I/R injury. One strategy to overcome the failure of individual treatments and to achieve optimized cardioprotection would be the administration of combined therapies.
Can Melatonin and Nicotinamide Mononucleotide Protect the Heart?
Melatonin — the same melatonin used for insomnia and improving sleep — is recognized for its antioxidant capacity as well as anti-inflammatory properties. Melatonin has been shown to have a cardioprotective impact in young animals as well as a positive impact on mitochondrial activity. The increased burden of cardiovascular diseases during aging has been related to the low levels of melatonin. However, whether increasing blood levels of melatonin can regulate ROS production or recover heart cell function in aged heart tissue following I/R injury remains unknown.
Nicotinamide adenine dinucleotide (NAD+) is an essential cofactor for numerous cellular metabolic processes and has a dominant role in energy production. But with age and heart damage, NAD+ levels decrease, which is accompanied by reduced mitochondrial energy production. Nicotinamide mononucleotide (NMN) is the main precursor of NAD+. Restoring NAD+ cellular content with NMN could improve age-related physiological dysfunction and various diseases.
The Combination of NMN and Melatonin is Cardioprotective
In this study, Hosseini and colleagues examined the combined effects of NMN and melatonin on cardioprotection and mitochondrial function in I/R injury of aged male rats. They administered an injection of NMN (100 mg/kg/d) every other day for 28 days before the I/R injury in aged rats and then added melatonin. They then isolated the hearts from these rats and, after creating an I/R injury, added melatonin to the fluid being pumped by the heart.
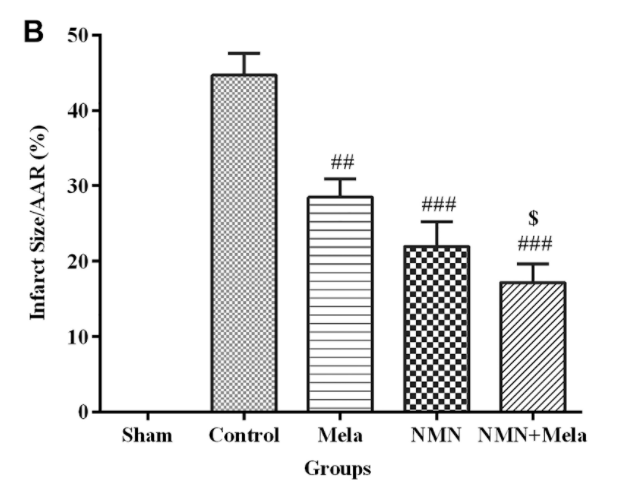
What they found was that the combination treatment of NMN and melatonin had an even greater increase in protective effects on the aged rat heart compared to the use of these compounds individually. For example, although NMN and melatonin individually could improve the ability of these hearts to pump blood and reduce the injury size, Hosseini and colleagues observed that the combination of the two yielded effects that were closer to levels seen in uninjured hearts.
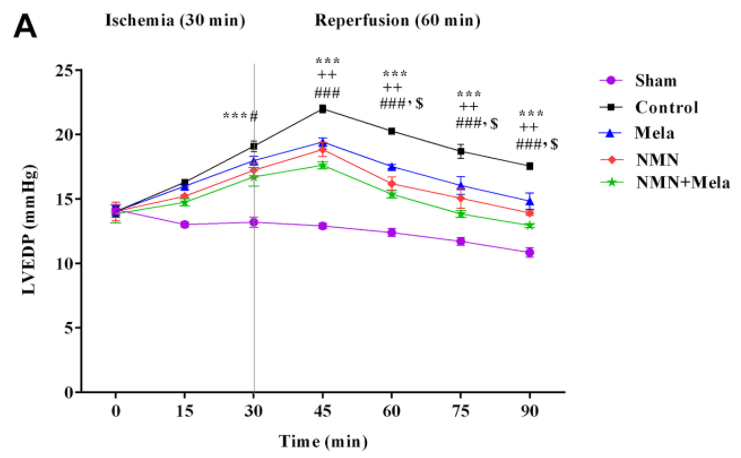
Similarly, the levels of lactate dehydrogenase (LDH), which is an indicator of heart cell damage that increases during I/R injury, were more impacted by the combination treatment of NMN and melatonin than by these compounds on their own. This was also the case regarding the heart’s oxidative status following I/R injury, as the combined therapy of NMN and melatonin had greater effects on the reduction of mitochondrial ROS and oxidative stress compared to using these treatments individually.
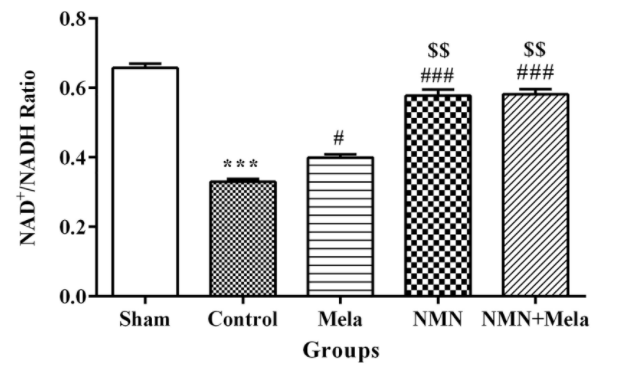
Regarding NAD+ levels, NAD+/NADH levels dropped upon I/R injury to levels that were about half of that seen in uninjured hearts. But the administration of NMN either individually or in combination with melatonin before ischemia could prevent the ischemia-induced decrease in NAD+/NADH ratio in the heart. Hosseini and colleagues propose that this suggests NMN enters the heart and boosts cellular NAD+ levels. Melatonin administration on its own had minimal albeit positive effects on the NAD+/NADH ratio, which isn’t surprising as it is not a precursor to NAD+.
These results show that a combination of NMN and melatonin in aged rat hearts may be promising for alleviating myocardial I/R injury and boosting cardioprotection during aging. Nevertheless, further studies are essential to illuminate the role of each of the multiple mechanisms contributing to cardioprotection. More importantly, these data do not provide any insight as to whether the effects of this combination treatment will translate to humans.

