Telomere Length and Aging
Telomere shortening is a well-known and widely-used marker for biological aging. But should it be?
DNA contains the instructions for life. Every time a cell replicates, it makes two copies of DNA inherited by each of the resulting two cells. Our DNA, and that of any animal and plant, is organized into strings called chromosomes. Because of this structure, every time chromosomes are copied, a tiny bit gets snipped off the end.
But this DNA trimming is not sustainable because genetic information would be lost with each cell doubling. This poses a problem. Since many cell doublings occur in every one of us, from the initial cell at conception to the trillions upon trillions that make up a person throughout a lifetime, our cells would be at risk of losing critical genetic information.
One solution devised by nature was to create the telomere — repetitive sequences of DNA that pad chromosomal tips. The telomere is the sacrificial DNA coil of the chromosome, becoming a bit shorter with each cellular replicating cycle but protecting the vital subjacent genetic information.
Some cells hardly replicate, so the telomere is sufficient for protecting the DNA integrity of these cells. For other cells, like those in development that are constantly replicating to make up the trillions upon trillions of cells that make up a human, it’s necessary to counteract the reproductive shortening of telomeres. In these cells, there’s an enzyme called telomerase that can activate to lengthen telomeres.
Critically short telomeres freeze up cells
There is a significant link between telomere length and a seemingly irreversible state when cells no longer grow or divide called senescence. Cells detect critically low telomere lengths and respond with senescence at a specific size of telomeres. Senescence is intimately linked to aging and the risk for age-related disease. When a tissue or organ can no longer be fixed or rejuvenated with new cells, it will deteriorate.
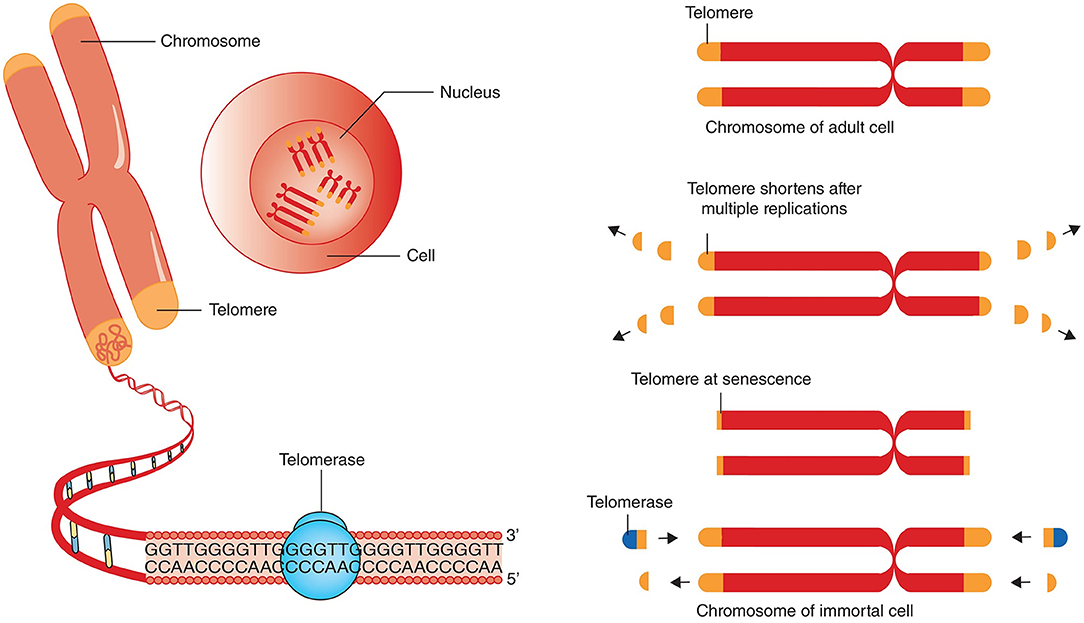
Chromosomes have repeated base segments called telomeres that shorten with each replication cycle (cell division). The enzyme telomerase can extend the telomere ends, thus prolonging cell life and potentially inducing immortality (a cancer cell hallmark).
How is telomere length measured in people?
Not all cells in the human body are accessible — you can’t just go in and lop off a piece of brain or heart to sample without causing damage. So, researchers have proposed the measurement of telomeres in immune cells called leukocytes as a surrogate marker for telomere shortening. Leukocyte telomere length (LTL) is often used in clinical studies.
How is telomere length related to aging?
Because telomeres typically decrease during a person’s lifespan, researchers have been on a mission to link the length of telomeres in different cells to biological age. Indeed, some studies show an association between telomere length and age. For example, with age, LTL was found to shorten with an average annual rate of 30–35 bp, reaching about 5–6 kb in people over 60 years old. This appears to be an important point in the context of current debates on whether human longevity has a maximal natural limit.
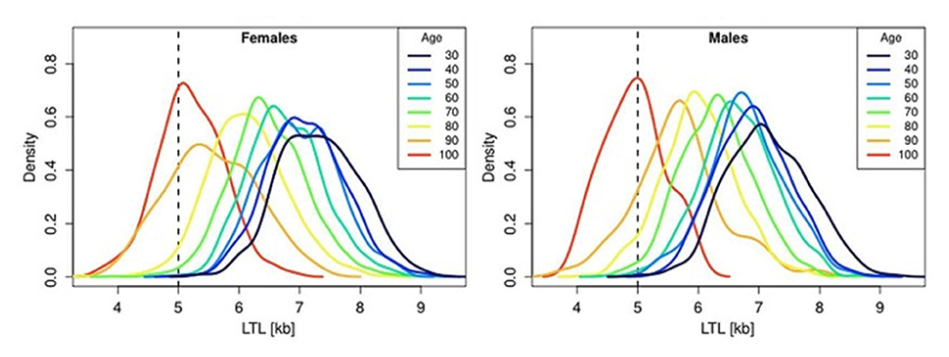
These plots show the LTLs for different age groups in males and females. Each colored line represents a different age group. The height represents the proportion of that age group with a particular LTL. As the age increases, the peaks shift toward the left, indicative of a greater proportion of people with shorter LTLs with increasing age.
How does telomere length relate to the risk of mortality?
Also, the findings from numerous population studies indicate that short telomeres are associated with higher morbidity and all-cause mortality and that telomere length appears to be a better predictor for survival than chronological age.
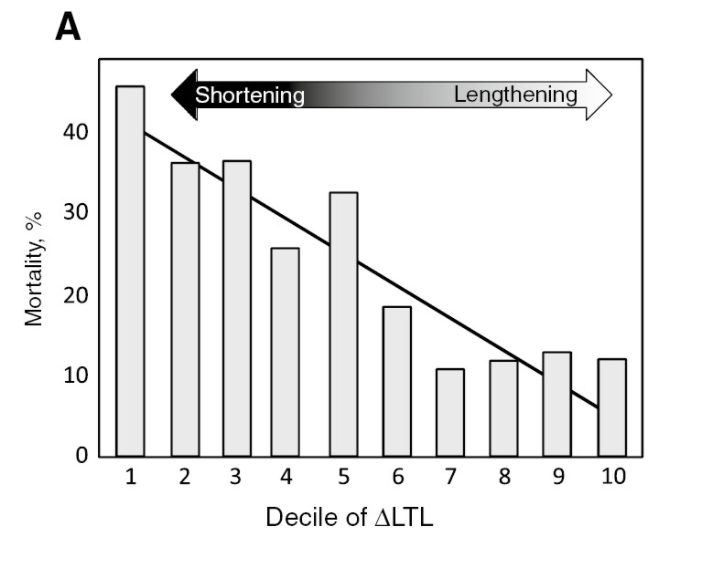
If you break up people into 10 groups based on changes in LTL over 5 years, those with increased LTL have a lower risk for mortality, whereas those whose LTL decreased have an increased risk.
How does telomere length relate to the risk of age-related diseases?
There is solid evidence linking telomere length with cardiovascular disease, particularly using LTL. Short telomeres promote atherosclerosis and impair the repair of vascular lesions. Alzheimer’s disease patients also have a reduced LTL.
Telomeres measured in tumor tissue from the breast, colon, and prostate are shorter than in healthy tissue from the same organ and patient. In healthy tissue directly adjacent to these tumors, telomeres are also shorter than in cells more distant from the cancerous lesion. A reduced telomere length in cancer tissue from the breast, colon, and prostate is associated with an advanced disease stage at diagnosis, faster disease progression, and poorer survival.
How does telomere length correlate with other hallmarks of aging?
When discussing the applicability of LTL as a reliable biomarker of aging, the comparison of LTL and other biomarkers of aging from the same individuals may certainly provide valuable information. Measurements of biological aging conducted either with telomere length based approaches or with other approaches, such as epigenetic clocks, could measure different aspects of the aging process.
In a recent study, nine measurements of biological age were assessed in a 20-year follow-up in 845 individuals from a Swedish population-based cohort. All measurements of biological age were more or less correlated with one another. Interestingly, the correlations among LTL and other biological age measurements were lower than among all other measurements. Therefore, it seems reasonable to use them together rather than separately.
How does increasing telomere length affect healthspan and lifespan?
Telomere lengthening by activating telomerase extends replicative lifespan in human cells. Telomeres longer than normal have been associated with diminished age-related diseases in humans. On the other hand, telomeres longer than normal have been associated with increased risks for increased blood pressure and a type of lung cancer.
Can telomere length be increased in humans?
There are many studies in which telomeres have been extended in laboratory animals using a variety of methods, such as genetic engineering techniques that activate telomerase.
The question is whether these kinds of studies can be translated into people. Along these lines, there are currently several studies where researchers are using gene therapy to deliver telomerase to prevent, delay, or even reverse aging and age-related diseases.
In addition, a recent study showed that hyperbaric oxygen chamber therapy can increase telomere length for the first time in humans. In this study, researchers found that repeated daily hyperbaric oxygen chamber therapy sessions increased the telomere length of certain immune cells called peripheral blood mononuclear cells by more than 20% in an aging population. What’s more, hyperbaric oxygen chamber therapy decreased the senescence of these cells by up to 37%.
Remaining telomere questions
The age-related telomere shortening phenomenon is highly complex, and biological mechanisms underlying this process are not yet definitively established. Whether telomere shortening reflects a cell division-based clock or is just a biomarker that transfers stress-associated signals to the cell is still debated.
Even though convincing evidence has been obtained that telomere length reflects cellular senescence levels in cells and animals studied in the lab, many clinical studies don’t support this. This inconsistency is so significant that some authors have even questioned whether the link between telomere length and aging-associated processes exists. Consequently, researchers debate whether telomere length is a reliable and valid tool to evaluate the rate of aging in human populations.
Also, doubts remain to what extent LTL reflects the situation in other organs and tissues. Until today, only few studies have explored the relationship between telomere length in different cell types. But the results are not so clear. Nevertheless, despite this uncertainty, telomere length, in particular LTL, remains one of the most widely used biomarkers of aging in human studies, clinical trials, and personalized medicine.

