Study Shows GDF-11 Protein from Young Marrow Alleviates Muscle Decline
Scientists find that transplanting prematurely aged mice with the bone marrow of young mice increases muscle mass while improving strength and endurance, a phenomenon largely replicated by GDF-11 treatment.
Highlights:
- Bone marrow from young mice increases muscle size and enhances the strength and endurance of prematurely aged mice.
- Young bone marrow elevates muscle regeneration, reduces cell death, and repairs damaged mitochondria.
- A protein called GDF-11 essentially replicates the benefits of young bone marrow transplantation.
Our muscles progressively deteriorate as we age, leading to sarcopenia, the age-related loss of muscle mass and strength. The capacity of our skeletal muscle to regenerate, which requires functional muscle stem cells, declines with age and contributes to sarcopenia. While stem cells are a potential treatment option for muscle decline, a new study from Yanbian University in China and Nagoya University in Japan suggests that the secretions of stem cells may work just as well.
As reported in the Journal of Cachexia, Sarcopenia and Muscle, young bone marrow transplantation prevents age-related muscle loss (atrophy) in a mouse model for aging. Inoue and colleagues show that recipients of young bone marrow transplants have increased muscle size and improved strength and endurance, mediated by reduced mitochondrial damage. Furthermore, a protein secreted by bone marrow called GDF-11 has similar effects.
Young Bone Marrow Reduces Muscle Dysfunction
A commonly used mouse model for aging is the SAMP10 mouse, genetically modified to age at about double the pace of normal mice. To achieve transplantation, Inoue and colleagues injected SAMP10 mice in the tibia bone with bone marrow from younger mice. The young bone marrow transplantation (YBMT) relieved the impaired running endurance and grip strength exhibited by the aged mice. Furthermore, YBMT increased the muscle mass and muscle cell size of the aged mice, indicating the prevention of atrophy.
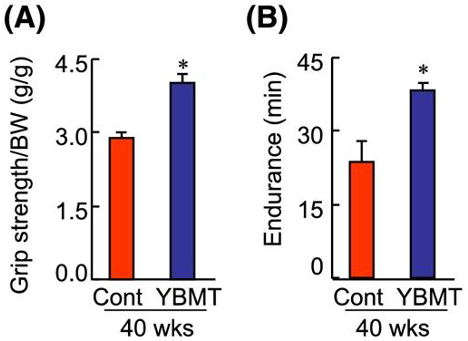
One of the reasons our muscles decline with age is because they lose their ability to regenerate. Regeneration, or growth in response to stress, requires muscle stem cells to become activated and proliferate. Muscle cell death also contributes to muscle decline and atrophy, leading to reductions in strength.
Inoue and colleagues found that markers for muscle stem cells increased in the muscles of YBMT mice. The muscles of YBMT mice also exhibited increased levels of a structural protein (desmin) that suggest increased regeneration. Additionally, markers of cell death were lower in YBMT muscle. These findings suggest that YBMT restores muscle regeneration capacity and reduces muscle cell death, which can explain the prevention of atrophy and weakness.
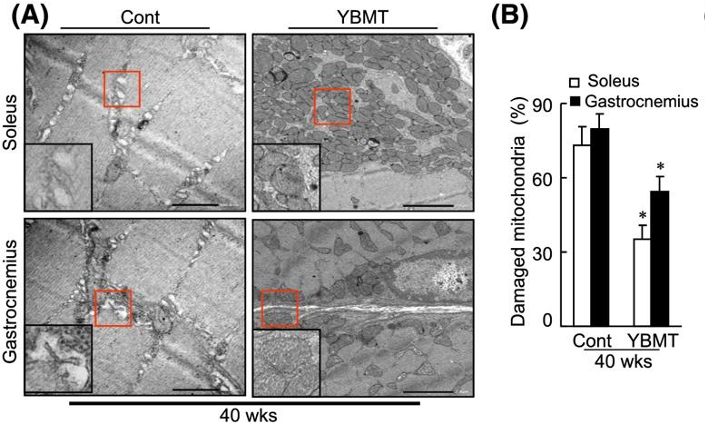
Healthy and functional mitochondria are foundational in meeting the high energy demands of muscle. Inoue and colleagues observed severe degeneration of mitochondria in the muscle of aged mice. However, YBMT lowered the number of damaged mitochondria, indicating that YBMT improves muscle mitochondrial degeneration.
Stem Cells or their Secretions for Anti-Aging?
Inoue and colleagues discovered that a protein called growth differentiation factor-11 (GDF-11), reported to decline with age, was elevated in YBMT mice. Because of this, the researchers supplemented aged mice with GDF-11, resulting in increased endurance, muscle cell size, regeneration, and decreased cell death. Thus, much of the anti-aging effects of YBMT seem to be mediated by GDF-11, purportedly secreted by stem cells in the bone marrow transplant.
While stem cells themselves seem like a promising therapy for regenerating tissues and reversing the aging process, there have been problems with stem cells grafting onto target tissues. Some scientists have now shifted their attention to the secretions of stem cells, such as GDP-11. These secretions seem to have the same effects as stem cells without problems like grafting. Several clinical trials are underway utilizing stem cell secretions (secretomes) for diseases like arthritis and stroke. Thus, stem cell secretomes may be an anti-aging therapy of the future.
Model: SAMP10 accelerated aging mice
Dose: weekly subcutaneous injections of 450 µg/kg GDF-11

