Study Finds NMN Protects Intestinal Wall from Cancer Radiation Therapy Injury
Chinese scientists find that NMN protects against intestinal damage caused by radiation — commonly used to treat cancer patients.
Highlights:
- NMN reduces DNA damage and reactive oxygen species (ROS) in intestinal cells exposed to radiation.
- In mice, treatment with NMN helps prevent structural impairments caused to the intestinal wall after radiation exposure.
- Pro-longevity enzymes called sirtuins mediate NMN’s benefits.
Radiotherapy is commonly used to treat elderly cancer patients who are often too frail to undergo surgery or receive chemotherapy. However, 60 to 80% of patients who receive radiotherapy for abdominal and pelvic tumors suffer from intestinal injury. Furthermore, there is currently no option available to rescue radiation-induced intestinal damage.
Now, researchers from the Chinese Academy of Medical Sciences and Peking Union Medical College report in Free Radical Biology and Medicine that NMN ameliorates radiation-induced damage in mice. In mouse intestinal cells deficient in a protein called NRF2, Zhao and colleagues show that NMN reduces DNA damage and decreases ROS invoked by radiation. Treatment with NMN also protects against radiation-induced intestinal injury in mice deficient in NRF2. The promising effects of NMN were mediated by increases in sirtuin enzymes.
NMN Protects the Intestinal Wall from Radiation
Radiation kills cancer cells by producing ROS and inducing DNA damage, but it can also cause damage to surrounding cells. The NRF2 protein plays an important role in reducing ROS and DNA damage but decreases with aging. Thus, NRF2 deficiency makes elderly individuals more susceptible to radiation-induced damage. For this reason, Zhao and colleagues experimented with NRF2-deficient cells to model what occurs with aging.
Not only did NRF2-deficient intestinal cells exposed to radiation display increased levels of ROS, DNA damage, and cell death, but they also had more senescent cells — cells that progress the aging process when not eliminated. These findings suggest that radiation may trigger senescence in cancer patients. Moreover, NMN treatment reduced ROS, DNA damage, and cell death. These findings suggest that NMN can help compensate for NRF2 deficiency in intestinal cells challenged with radiation.
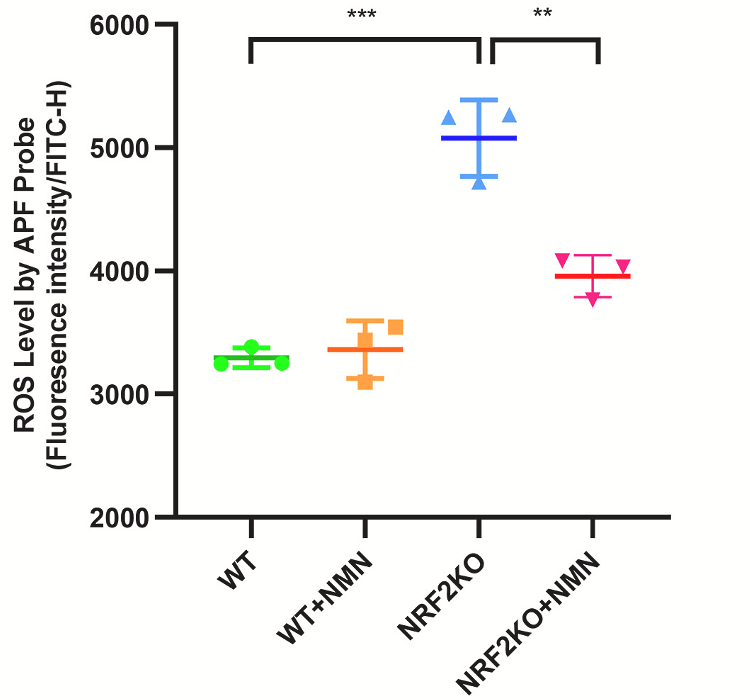
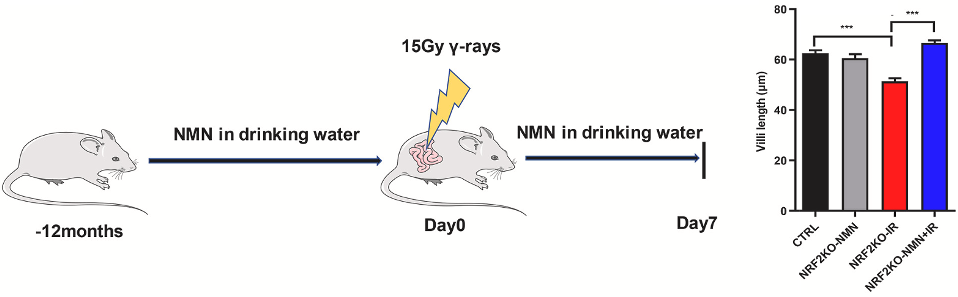
Sirtuins are enzymes that use NAD+ to fuel cell survival activity. Zhao and colleagues showed that NMN increased sirtuin protein levels, suggesting NMN’s effects are mediated by sirtuins. They then tested whether NMN and sirtuins protect against radiation-induced intestinal damage by feeding NRF2-deficient mice 300 mg/kg/day of NMN. The mice were then exposed to abdominal radiation.
Intestinal damage was measured by assessing several features of the intestinal wall, including villi length. Villi are outgrowths of the intestinal wall that help to increase the surface area of the intestine for nutrient absorption. While radiation exposure led to decreased villi length in untreated NRF2-deficient mice, when these mice were treated with NMN, villi length was restored. These findings show that NMN protects against radiation-induced intestinal damage in cases of low NRF2.
Previous studies have shown that NMN promotes the function of sirtuin 1 (SIRT1). However, there exists a family of seven sirtuins, and in this study, NMN increased sirtuin 6 (SIRT6) and sirtuin 7 (SIRT7). These sirtuins seem to have antioxidant properties, as they mediate the reduction in ROS — oxidants that can cause damage to cells. Sirtuins also mediate DNA repair processes.
“Our results reveal the function of SIRT6 and SIRT7 independent of NRF2 and implicate the sophisticated network of sirtuin family [members] as regulators of different physiological or pathological states,” say the authors.
NMN Reduces Cancer Treatment Side Effects and Enhances Efficacy
Cancer treatments are far from perfect and come with long-term side effects. However, some of these side effects are prevented by NMN. For example, NMN combined with troxerutin was shown to fully protect the heart from damage caused by the chemotherapy drug doxorubicin. Now, Zhao and colleagues show that NMN can alleviate the long-term side effects of radiation therapy.
“NMN could be a promising supplement to alleviate radiation-induced intestinal injury,” state the authors.
NMN also enhances the effects of immunotherapies, the latest innovation in cancer therapy that utilizes immune cells to suppress tumor progression. For example, NMN was shown to enhance natural killer cell immunotherapy against skin and liver cancer and boosts CAR-T cell immunotherapy against blood cancer. Furthermore, the NAD+ precursor NR has also been shown to enhance immunotherapies and NRH prevents tumor regrowth after brain cancer surgery. Thus, boosting NAD+ with its precursors holds promise in conjunction with traditional cancer treatments.
Model: NRF2-deficient mice exposed to radiation
Dosage: 300 mg/kg/day oral NMN

