New Study Shows that Supplement Spermidine Slows Down Liver Aging
Spermidine improves liver cell viability under chemical-induced toxicity with an increase of mitochondria.
Highlights
- Treatment with spermidine increases liver cell viability in the presence of calcium chloride.
- Feeding spermidine to mice also improves their liver tissue integrity under dietary conditions that promote scarring.
- Spermidine promotes cell mitochondria generation along with diminishing harmful oxidative stress in cells.
As we age, our livers accumulate scar tissue, driving a condition called liver fibrosis. With this physiological complication comes substantial deterioration of a tissue layer called the endothelium, facilitating a snowball effect of liver damage. Researchers have previously turned to boosting nicotinamide adenine dinucleotide (NAD) – an essential molecule for cell energy generation – to combat liver fibrosis. However, spermidine, a naturally occurring molecule present in all plant and animal tissues, has emerged as a possible pharmaceutical agent to allay age-related liver ailments.
As published in Nutrients, Hernández and colleagues from the University of Barcelona in Spain show that 3 mM of spermidine fed to mice in drinking water protects liver endothelium from liver fibrosis by enhancing the genesis of mitochondria and reducing oxidative stress. When fed spermidine, two mouse models for liver disease, calcium chloride (CCl4)-fed mice and mice fed a diet that induces fatty liver disease, had better liver tissue integrity. What’s more, treating mouse and scarred human liver cells with spermidine improved cell viability and increased mitochondrial DNA copy number – an indicator of higher numbers of mitochondria. Not only that, but mouse livers exhibited substantially less cell damaging oxidative stress when treated with spermidine. These results support the notion that spermidine may be beneficial to prevent age-related liver disease and enhance overall liver health as we age.
Spermidine Maintains Liver Endothelial Health Under Toxic Conditions
To demonstrate that spermidine preserves liver endothelium from oxidative stress, Hernández and colleagues treated mouse and human liver endothelial cells – cells that makeup liver endothelium – with hydrogen peroxide (H2O2), which mimics oxidative stress. Oxidative stress decreased liver endothelial cell viability by about 50% but recovered to about 90% when spermidine was added. These results showcase that spermidine preserves the survival of liver endothelial cells under oxidative stress.
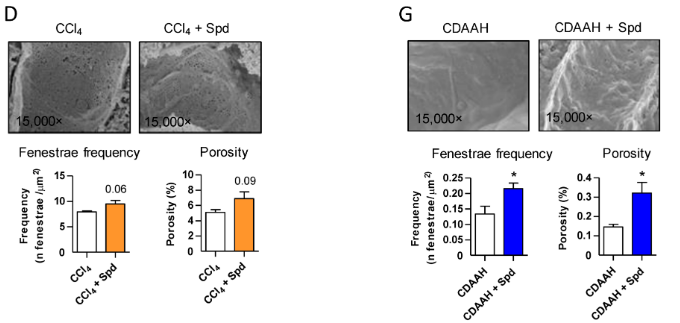
Hernández and colleagues went on to examine endothelial liver tissue integrity, an indicator of liver fibrosis, in the mouse CCl4-fed toxic liver disease model to the fatty liver disease mouse model. They found that spermidine addition under CCl4 conditions increased liver fenestrae, part of the liver’s filtration system, by approximately 40% in the toxic liver model and 77% in the fatty liver model, marking an improvement in liver tissue health. These findings show that spermidine preserves liver endothelium under fibrotic or scarring conditions.
Spermidine Alleviates Dead Liver Cell Accumulation
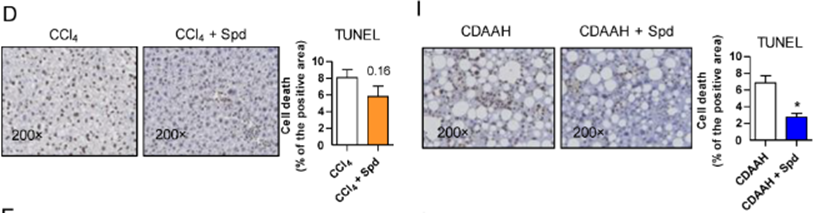
To further examine tissue scarring, Hernández and colleagues directly examined scar tissue in the liver disease mouse models. The researchers found that while spermidine failed to reduce the accumulation of scar tissue, it diminished the buildup of dead cells by ~38% in the toxic liver model and ~71% in fatty liver model. These results indicate that while spermidine did not significantly reduce tissue scarring, it substantially decreased cell death under fibrosis-promoting conditions.
Spermidine Enhances Mitochondrial Genesis in Fibrotic Livers
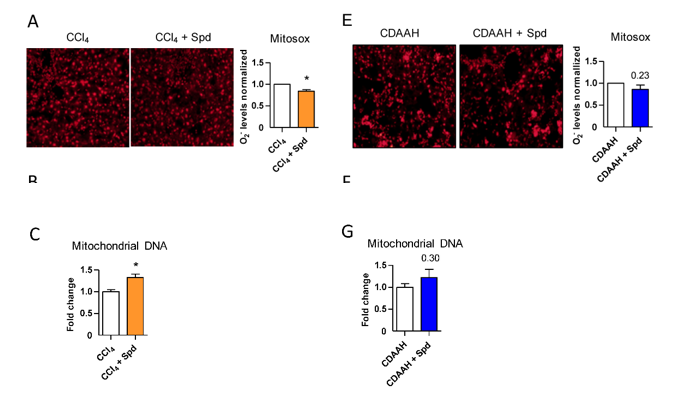
Hernández and colleagues then stained mouse endothelial cells with antibodies to find out how fibrosis-promoting oxidative stress conditions (H2O2) affects the presence of power-generating organelles within cells called mitochondria. The research team found that H2O2 dramatically reduces mitochondrial DNA, an indicator of diminished mitochondrial abundance. The addition of spermidine to these cells significantly improved mitochondrial DNA levels. Under fatty liver disease-promoting conditions, the addition of spermidine also significantly enhanced the concentration of mitochondrial DNA. These findings indicate that spermidine preserves endothelial cell power-generating capacity by increasing mitochondria numbers.
.
Searching for Details About Spermidine’s Mechanism of Action
The University of Barcelona-based researchers showed for the first time that spermidine not only prevents degeneration of liver tissue under toxic conditions but also maintains endothelium health by restoring the number of mitochondria. What’s more, spermidine acts to diminish oxidative stress by reducing the accumulation of reactive oxygen species, namely superoxide.
Questions remain regarding the precise mechanism by which spermidine attenuates the progression of fibrosis in mice. However, the study confirms previous findings pointing to spermidine reducing reactive oxygen species accumulation in endothelial cells. A big question confronting research is whether spermidine usage has the same effects in humans. Also, once scientists figure out more precise mechanisms of action by which spermidine exerts its effects, we will be able to more accurately target liver ailments with potentially newly-devised molecules for pharmaceutical usage.

