Shocking Study Reveals Common Cough Medicine Could Treat Lung Disease
Dextromethorphan (DXM), a common cough medicine, significantly reduces lung fibrosis in mice, offering new hope for over 250,000 Americans living with the disease.
Highlights
- Dextromethorphan (DXM), a common cough suppressant, significantly reduced lung fibrosis (tissue scarring) across multiple models.
- Over 250,000 Americans live with pulmonary (lung) fibrosis, which has a survival rate of only 48.4% after 10 years for elderly patients.
Idiopathic pulmonary fibrosis (IPF), the most prevalent form of fibrosis with an unknown cause, impacts about 1 in 200 adults aged 70 and older in the United States. Currently, more than 250,000 Americans live with pulmonary fibrosis, with 50,000 new cases diagnosed each year. The disease causes scar tissue to develop in the lungs, impairing oxygen transfer from air sacs to the bloodstream. As scarring worsens, patients often struggle with shortness of breath, particularly during physical activity, along with symptoms such as chronic fatigue, persistent coughing, and anxiety. These effects, compounded by the lack of curative treatments, emphasize the need for new therapeutic approaches.
In response to this unmet need, a recent study published in Science Translational Medicine identified dextromethorphan (DXM), a widely used cough suppressant, as a potential antifibrotic treatment. The study explores how DXM might reduce lung fibrosis by inhibiting the buildup of collagen, a key driver of scarring in fibrotic tissues. This discovery opens new possibilities for targeting fibrotic diseases, especially among older adults vulnerable to its progression.
The Connection Between Fibrosis and Aging
As individuals age, their tissues undergo gradual changes, increasing susceptibility to fibrotic diseases. These changes, including increased inflammation and cellular damage, affect several organs, including the lungs, liver, kidneys, and skin, leading to a progressive decline in organ function. In particular, lung fibrosis results in decreased respiratory capacity and increased mortality rates among the elderly.
According to a study on IPF mortality, the survival rate for elderly patients drops to about 48.4% within 10 years of diagnosis. The all-cause mortality rate reaches 97.6 per 1,000 person-years for male patients and 65.9 per 1,000 person-years for female patients, with respiratory failure as the leading cause of death. Although current treatments, such as pirfenidone and nintedanib, can slow the progression of the disease, they do not provide a cure. Consequently, the search for more effective treatments continues.
Dextromethorphan Mitigates Collagen Buildup and Fibrotic Progression
Since collagen buildup drives the progression of fibrosis, the study evaluated whether DXM could reduce collagen deposition in the extracellular matrix (ECM). Collagen type I (COL1), a major structural protein in the ECM, contributes to fibrosis by promoting tissue scarring and stiffness.
The study tested dextromethorphan (DXM) on human lung fibroblasts stimulated with TGF-β1 to mimic fibrotic conditions. DXM treatment reduced the buildup of collagen type I (COL1), a key contributor to fibrosis. Additional tests confirmed that DXM decreased COL1 accumulation in human lung, kidney, and skin cells. In precision-cut lung slices, DXM also reduced collagen deposition over two weeks, reinforcing its antifibrotic potential in a physiologically relevant tissue model.
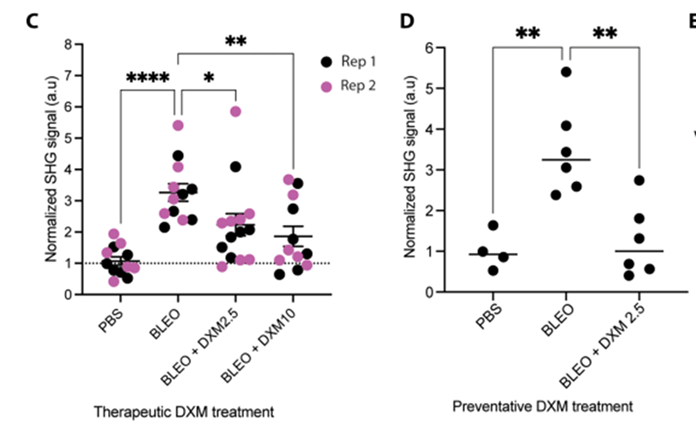
Building on the in vitro and ex vivo results, researchers evaluated DXM’s efficacy in an in vivo mouse model of lung fibrosis. Animals were administered DXM either preventatively or therapeutically. In the preventative regimen, mice received 2.5 mg/kg DXM daily from day 1 to day 20 following bleomycin treatment. In the therapeutic regimen, animals were treated with either 2.5 mg/kg or 10 mg/kg DXM from day 7 to day 20. Both treatment protocols resulted in a notable reduction in fibrillar collagen content within lung tissue, accompanied by decreased scarring and improved tissue structure.
Researchers also found that therapeutic DXM administration at 2.5 mg/kg reduced the expression of key profibrotic markers. Myofibroblast differentiation, the process where fibroblasts transform into collagen-producing myofibroblasts that drive tissue scarring and stiffness, was also suppressed by DXM. By preventing this transformation, DXM helps limit the buildup of collagen and the progression of fibrosis.
Together, these results provide strong evidence for DXM’s antifibrotic activity across multiple experimental models, highlighting its potential as both a preventative and therapeutic intervention for pulmonary fibrosis.
Future Prospects for DXM in Fibrosis Treatment
Given the strong link between aging and fibrosis, DXM’s ability to reduce fibrotic burden holds promise for improving the quality of life for elderly individuals. Its accessibility and established safety profile make it an appealing candidate for clinical development. However, further validation through clinical trials is needed to confirm its safety and efficacy, along with additional research to determine its applicability to other age-related fibrotic conditions. By targeting collagen buildup and ECM remodeling, DXM offers a novel avenue for addressing fibrosis in patients with few existing treatment options.
Model: bleomycin-induced lung fibrosis model in C57BL/6J mice
Dosage: Preventative regimen: 2.5 mg/kg per day, from day 1 to day 20
Therapeutic regimen: 2.5 mg/kg or 10 mg/kg per day, from day 7 to day 20

