Senolytics Alleviate COVID-Induced Brain Aging and Lifespan Shortening in New Study
Scientists find that senolytics reduce senescent (aged) cells in a human COVID-19 brain model and significantly increase the lifespan of COVID-19 mice.
Highlights:
- In aged brains grown from human cells, the senolytics navitoclax, ABT-737, and dasatinib + quercetin (D+Q) reduce senescent cells, while only D+Q reverses biological age.
- COVID-19 patient brains exhibit increased senescence, and navitoclax, ABT-737, and D+Q reduce senescence in SARS-CoV-2 infected artificial human brains.
- The senolytics D+Q and fisetin, but not navitoclax increase the lifespan of SARS-CoV-2 infected mice by 60%.
While scientists are still unraveling the cellular sources of brain aging, senescent cells seem to be a major contributor. Senescent cells accumulate with age and promote chronic inflammation — a key underlier of aging that progressively damages tissues. Now, researchers from institutions in Australia, Russia, Germany, Chile, the U.K., and the U.S., including Harvard, have found that COVID-19 induces brain senescence — a phenomenon that can be reversed.
Aguado and colleagues report in a preprint (not peer-reviewed) from BioRxiv that senolytic therapy alleviates brain aging and COVID-19 neuropathy. They show that senolytics reverse the biological age of brains grown from human cells while reducing senescent cells generated by SARS-CoV-2 infection. Furthermore, senolytics prevent neurodegeneration and increase the lifespan of SARS-CoV-2-infected mice.
“Given that senescent cell clearance results in reversal of the aging process, these findings support an important role for senescent cells in driving human brain aging,” state the authors.
Senolytics Reverse Brain Aging
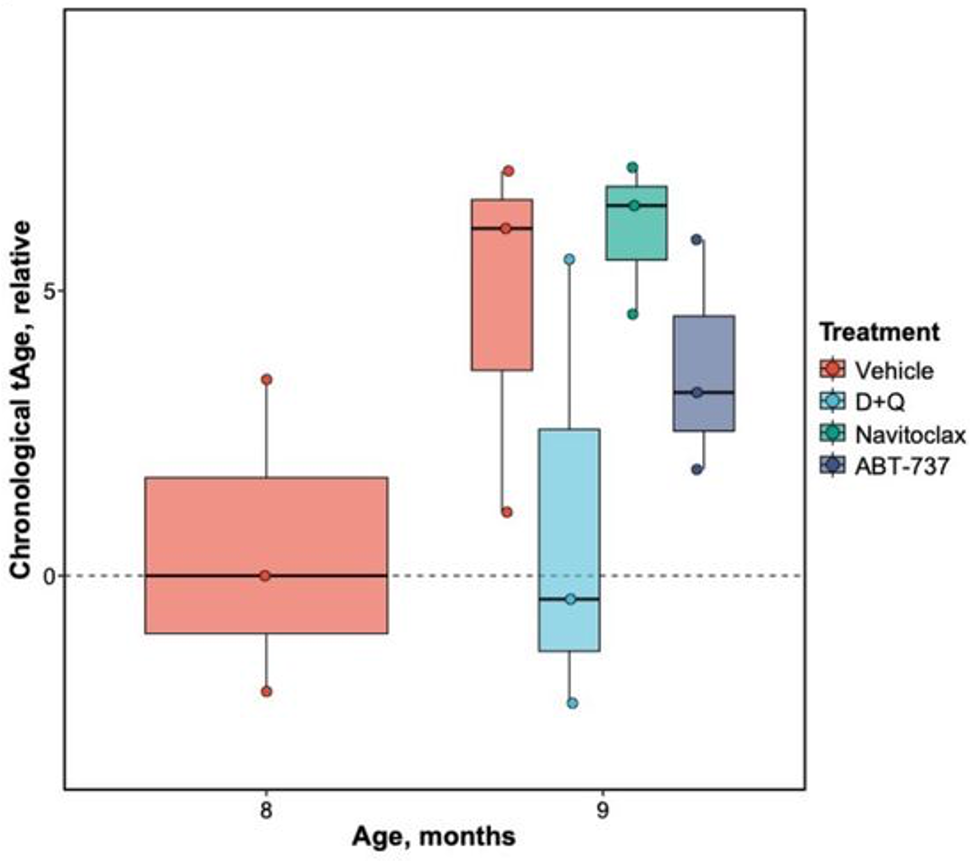
Organoids are artificially grown miniature organs. To study the effect of senolytics on brain aging, Aguado and colleagues generated brain organoids from human stem cells and aged them. They tested three senolytics (known to selectively eliminate senescent cells), navitoclax, ABT-737, and D+Q. Exposure to all three senolytics leads to a reduction in senescent cells.
Furthermore, age clock predictions based on gene activity showed that D+Q made 9-month organoids one month younger, which was not seen with the other two senolytics. D+Q was also negatively associated with aging genes and positively correlated with lifespan-extending interventions, such as caloric restriction and rapamycin administration.
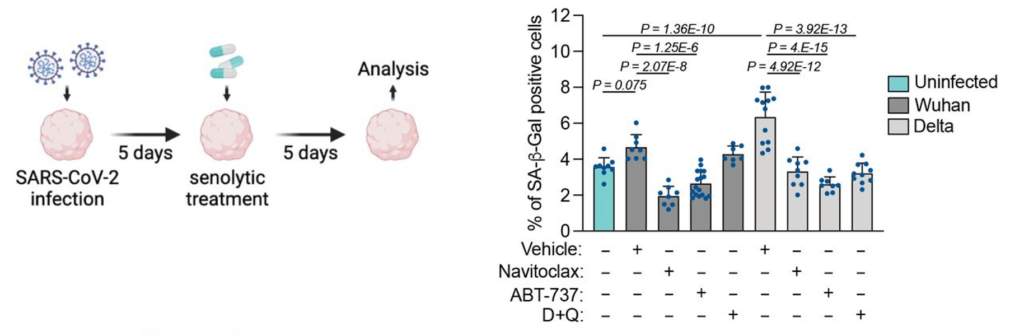
To determine the effect of COVID-19 on brain aging, Aguado and colleagues examined post-mortem brains from COVID-19 patients, where they found an increase in senescence markers. They next infected human brain organoids with different viral pathogens, including seven SARS-CoV-2 variants. Most variants elicited increases in senescence, with the delta variant showing the strongest induction. All three senolytic treatments reduced senescent cells in COVID-19-infected human brain organoids and reduced viral load up to 40-fold, suggesting that senescent cells harbor viruses.
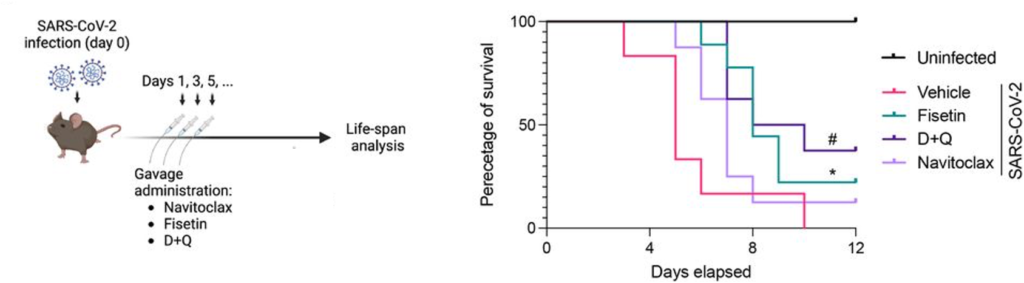
Aguado and colleagues next tested the effects of the senolytics navitoclax, fisetin, and D+Q on mice with COVID-19, which normally live for only about five days. The senolytics were administered orally every two days. Treatment with D+Q or fisetin improved survival and extended median lifespan by 60%. By the end of the 12-day experiment, 22% of the fisetin-treated, 38% of the D+Q-treated, and 13% of navitoclax-treated mice were still alive.
COVID-19 can lead to long-term neurological deficits in humans, including problems with coordination and memory. By examining the brains of COVID-19 mice, Aguado and colleagues found that SARS-CoV-2 infection leads to neurodegeneration (loss of neurons). However, this neurodegeneration was reduced by all three senolytics (navitoclax, fisetin, and D+Q), suggesting that COVID-19 contributes to neurodegeneration by triggering senescence.
Decelerating COVID-Induced Aging
Some COVID-19 survivors exhibit symptoms surprisingly similar to aging long after viral infection, sometimes termed Long COVID. Whether or not COVID accelerates aging in multiple organs remains unclear, but senescent cells have previously been observed in the lungs of COVID-19 patients. And now, Aguado and colleagues have shown that senescent cells are rampant in COVID-19 patient brains.
If senescent cells are indeed responsible for the aging-like symptoms exhibited by COVID-19 survivors, it may be possible to alleviate their aging effects with senolytics. However, there are not any clinical studies showing that this may be true.
Model: Female K18-hACE2 transgenic mice infected with SARS-CoV-2 (COVID-19 model)
Dosage (oral): 100 mg/kg navitoclax, 5 mg/kg dasatinib + 50 mg/kg quercetin, or 100 mg/kg fisetin every other day for twelve days or until death

