Scientists Reliably Predict Age by Measuring Blood Proteins
A Stanford University study found protein signatures for people of different age groups
Highlights
· Protein levels in people’s blood can predict their age
· Aging isn’t a smoothly continuous process.
Through some Frankenstein-like experiments of exchanging blood between young and old animals, scientists have thought for some time that age-related changes of compounds in blood could provide new insights into age-related disease biology. After all, aging is a predominant risk factor for several chronic diseases that limit health span, so finding markers and targets in the blood that inform and mitigate aging is a prudent endeavor.
Lehallier and colleagues from Stanford University measured nearly 3,000 plasma proteins from thousands of adults aged 18 to 95 years old, finding that there are age-related waves of complex changes in the protein composition of human plasma — the liquid, not cellular, component of blood. Published in Nature Medicine, this study of aging identified unexpected signatures and pathways that might offer potential targets for age-related diseases.
Age-Related Particles Flow-Through Blood
Researchers have had a tough time trying to pin down bits of our DNA that affect aging and longevity. But the protein composition of cells, bodily fluids, and tissues changes similarly with age and provides insights into complex biological processes, as proteins are often direct regulators of cellular pathways.
In particular, blood, which contains proteins from nearly every cell and tissue, has been analyzed to discover molecules that could influence aging. Along these lines, the aging of animals results in changes in the blood’s protein composition that reflect aspects of the aging of different cell types and tissues.
Perhaps the strongest evidence that blood can be used to study aging comes from experiments employing heterochronic parabiosis, which is a surgically induced state that connects the circulatory systems of young and old mice. These studies show that multiple tissues, including muscle, liver, heart, pancreas, kidney, bone, and brain, can be rejuvenated in old mice.
Plasma from old mice is sufficient to accelerate brain aging after infusion into young mice, and young plasma can reverse aspects of brain aging. Together, these studies support the notion that the protein composition of plasma harbors key regulators of aging and that identifying protein signatures may help in understanding how we age.
Predicting Age from a Drop of Blood
In this study, Lehallier and colleagues analyzed the protein composition of plasma from young adults to people in their 90s. They measured 2,925 plasma proteins from 4,263 young adults to nonagenarians (18 to 95 years old).
“Proteins are the workhorses of the body’s constituent cells, and when their relative levels undergo substantial changes, it means you’ve changed, too,” said senior author Tony Wyss-Coray, Ph.D., professor of neurology and neurological sciences, the D. H. Chen Professor II and co-director of the Stanford Alzheimer’s Disease Research Center.
“Looking at thousands of them in plasma gives you a snapshot of what’s going on throughout the body.”
They discovered changes in the levels for 1,379 proteins across the lifespan and linked these changes to biological pathways and disease.
“We’ve known for a long time that measuring certain proteins in the blood can give you information about a person’s health status — lipoproteins for cardiovascular health, for example,” said Dr. Wyss-Coray.
“But it hasn’t been appreciated that so many different proteins’ levels — roughly a third of all the ones we looked at — change markedly with advancing age.”
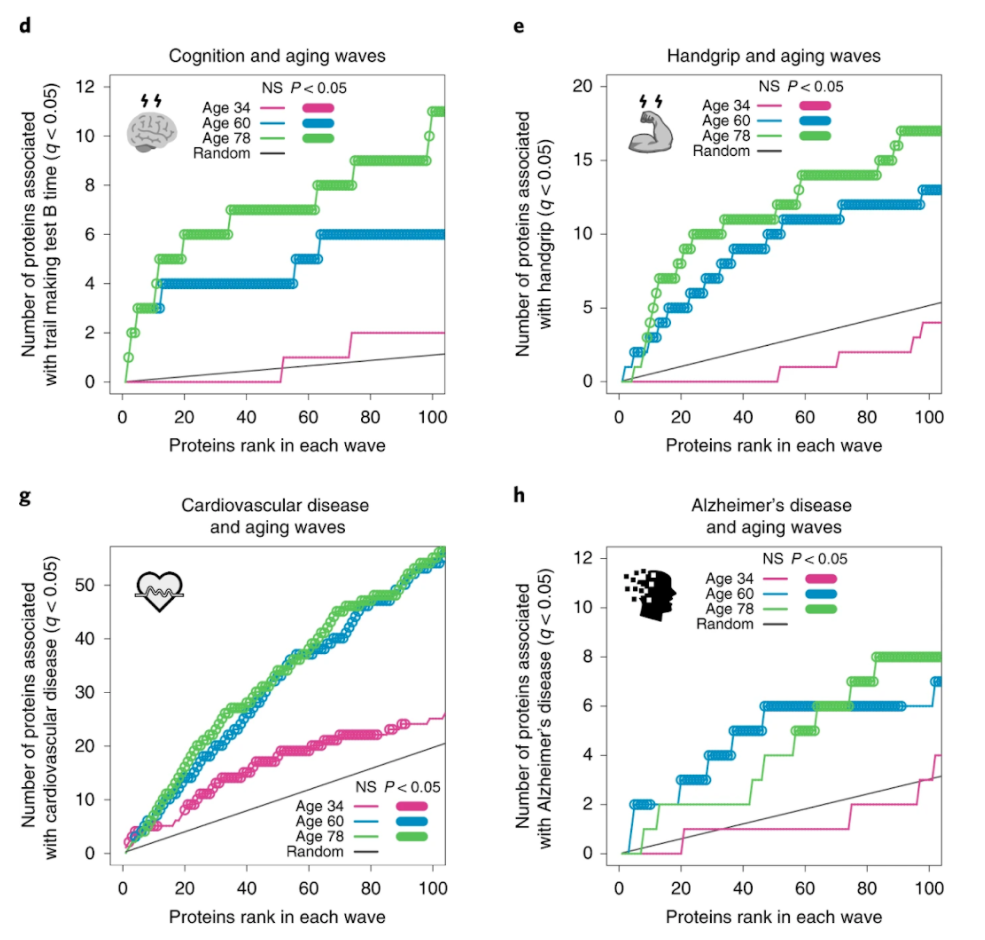
Lehallier and colleagues saw waves of changes in the plasma’s protein composition in the fourth, seventh, and eighth decades of life. These age-related protein signatures reflected distinct biological pathways and were linked to age-related diseases and traits.
For example, individuals who were predicted to be younger than their chronological age performed better on cognitive and physical tests.
“We had data on hand-grip strength and cognitive function for that group of people,” Wyss-Coray.
“Those with stronger hand grips and better-measured cognition were estimated by our plasma-protein clock to be younger than they actually were.”
Men and Women Have Different Age-Related Proteins
It is well known that men and women age differently, yet, surprisingly, Lehallier and colleagues found that two-thirds of proteins that changed with age also changed with sex (895 of 1,379 proteins). This means that a unique proteomic clock can be used to predict age in men and women and deviations from this plasma proteomic clock are linked with changes in age-related diseases and traits.
“The differences were striking,” Wyss-Coray said.
He added that this finding strongly supports the rationale for the National Institutes of Health’s policy, instituted in 2016, promoting increased inclusion of women in clinical trials and the demarcating of sex as a biological variable.
Creating an Applicable Aging Clock
Lehallier and colleagues were able to zero in on a panel of 373 proteins that can be used to assess the relative health of an individual and to measure healthspan. All of this from just a drop of blood.
In fact, a mere nine proteins were enough to do a passable job, Wyss-Coray said.
“After nine or 10 proteins, adding more proteins to the clock improves its prediction accuracy only a bit more,” he said.
“With machine learning, you could potentially make a test with good accuracy based on just those nine proteins.”
More large-scale plasma proteomic studies are required to establish the validity and utility of this “clock” and whether specific protein subsets are more appropriate to reflect particular clinical and functional parameters.

