Scientists Identify Transporter for Cell Absorption of NMN in Mice
Washington University School of Medicine in St. Louis researchers identify a nicotinamide mononucleotide (NMN)-specific transporter called Slc12a8 in mice.
Nicotinamide mononucleotide (NMN) is a precursor that cells use to synthesize the essential molecule NAD+ and is proposed to counteract age-associated diseases and disorders associated with a decline in tissue NAD+ levels.1-14 But how NMN is taken up into cells has not been entirely clear.
In a study published in Nature Metabolism, researchers from the Washington University School of Medicine identify a gene called Slc12a8 that encodes a specific NMN transporter. Led by Alessia Grozio, the research team found that Slc12a8 is present in high amounts in the mouse small intestine. This transporter appears to exists in humans, mice, zebrafish, fruit flies, and roundworms. When deleted, NMN was no longer transported into mouse cells, both those in culture and in live animals.
“Our work identifies the first NMN transporter and demonstrates that Slc12a8 has a critical role in regulating intestinal NAD+ metabolism,” said Grozio and colleagues.
How Does the Body Absorb NMN?
NMN has been proposed as a therapeutic agent in age-associated disease prevention. Scientists find declining NAD+ levels during aging in many tissues, including muscle, liver, fat (adipose tissue), brain, pancreas, spleen, heart, kidney, and lung, which contribute to age-associated diseases. Studies have reported that NMN has positive effects in improvement of diseases and combating age-associated physiological decline.1-14
NMN is quickly absorbed into tissues and cells where it is used for the synthesis of an essential molecule called nicotinamide adenine dinucleotide (NAD+). That’s why leading scientists have proposed an effective transporter exists in the body for direct absorption of NMN into tissues and organs. NMN absorbed from the gut enters blood circulation within two to three minutes and is transported to tissues within 10-30 minutes following ingestion.5,7,14 NMN is then immediately used for NAD+ biosynthesis and significantly increases NAD+ concentration in cells over the course of 60 minutes following ingestion.5 However, how NMN is transported into cells and tissues remains incompletely understood.
Slc12a8 Is an NMN Transporter
To identify the proposed NMN transporter, Grozio and colleagues analyzed gene activity in a variety of tissues, including the liver, pancreas, and brain. Through a series of experiments to pin down molecules involved in the shuttling of NMN into cells, the Washington University School of Medicine team discovered one gene for a transporter called Slc12a8. The team found high gene activity levels of the Slc12a8 transporter in the small intestine and pancreas of the mice and moderate levels in the liver and fat tissue.
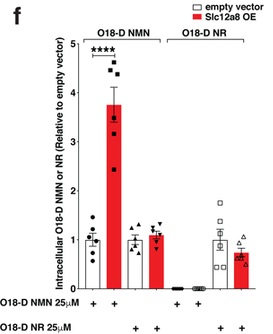
The scientists ran further experimentation to confirm the Slc12a8 gene encodes a transporter for NMN and not another NAD+ precursor. One way they did so was by blocking the entry of another NAD+ biosynthesis precursor nicotinamide riboside (NR) into liver cells and looking for increases in NAD+ levels upon NMN supplementation. Cells that were given NMN and an inhibitor for NR uptake had major increases in NMN within minutes, suggesting the presence of this NMN transporter. Importantly, when they genetically decreased levels of Slc12a8, this fast intake of NMN to the cell was lost.
For further evidence confirming the Slc12a8 gene encodes the NMN transporter, the team of scientists inserted the transported into cells called NIH3T3 cells. These cells have a very weak uptake of NMN under normal circumstances. By increasing the Slc12a8 transporter gene, the NIH3T3 cells had much higher levels of NMN in cells compared to their unmodified counterparts.
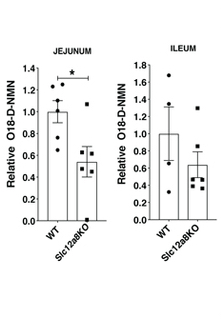
Grozio and colleagues also looked at whether Slc12a8 functions as a transporter for NMN cellular uptake in live mice. By reducing the levels of Slc12a8 in live mice, the researchers found reduced NMN cellular intake following NMN administration. Interestingly, the research team saw that in old mice Slc12a8 levels were increased in the aged mouse gut. They think that this tries to offset the declining levels of NAD+ seen with aging.
“The identification and the characterization of the Slc12a8 NMN transporter further advances our understanding of the physiological importance of NMN as a key systemic NAD+ intermediate,” concluded Grozio and colleagues. “Because NMN conveys remarkable effects of mitigating age-associated physiological decline in mice 4,15, identifying compounds that could promote the NMN-transporting function of the Slc12a8 protein will provide an interesting opportunity to develop a more effective anti-aging intervention, combined with NMN administration.

