Scientists Engineer Peptide-Blood Gels that Successfully Regenerate Bone
University of Nottingham researchers engineer peptide-blood gels that mimic natural healing, enhance bone regeneration, and reduce inflammation.
Highlights:
- Peptide-blood gels recreate the regenerative hematoma (RH) microenvironment, promoting cell recruitment and controlled growth factor release to accelerate healing and regeneration.
- Peptide-blood gels enhance bone regeneration and reduce markers of inflammation.
Whether you pull a muscle, stub a toe, or break a bone, the body activates efficient healing mechanisms to restore tissue integrity, reduce inflammation, and rebuild damaged structures. For major injuries, the body kickstarts the healing process by using liquid blood to form a regenerative hematoma (RH) – an enriched matrix containing a milieu of functional biomolecules that stimulate healing and regeneration. Notably, increasing evidence suggests we can manipulate the RH microenvironment to enhance its regenerative potential, making it a primary target for regenerative therapeutics.
Now, in a study published in Advanced Materials, researchers from the University of Nottingham engineered a biomaterial that replicates the properties of the RH using a combination of blood and peptide amphiphiles (PAs) – synthetic molecules that form nanostructures. This gel-like material not only mimics the compositional and functional properties of the natural hematoma but also enhances its ability to heal tissue.
Addressing Age-Related Declines in Healing
As the body ages, the regenerative capacity of tissues diminishes, leading to slower wound healing, decreased bone density, and increased susceptibility to chronic inflammation, a hallmark of aging. Peptide-blood gels could potentially counteract these age-related challenges by creating a microenvironment that mimics youthful healing responses, offering new hope for aging populations dealing with musculoskeletal injuries and chronic conditions.
Peptide-Blood Gels Recruit and Release Healing Molecules
The peptide-blood gel recreates the dynamic microenvironment of the RH by preserving key blood components and regulating the release of critical growth factors. When PAs were mixed with blood, they co-assembled with fibrin and other blood proteins to form a stable hydrogel. Results showed that this material stored and gradually released biomolecules like VEGF, TGF-β, and PDGF, demonstrating that the gel successfully replicates the natural RH response.
Moreover, laboratory tests revealed that the gel attracted and supported the growth of mesenchymal stromal cells, endothelial cells, and fibroblasts – cell types that modulate critical healing mechanisms. Uniquely, the cells adhered to and migrated into the gel, closely mimicking how natural RH promotes cell recruitment.
More importantly, the research team noted that the gels released growth factors in a controlled and sustained manner, ensuring a consistent supply of signals essential for regeneration. This prolonged-release contrasts with the rapid depletion seen in natural clots, enhancing the gel’s ability to support processes like angiogenesis, matrix deposition, and cell proliferation over an extended period. Collectively, these findings highlight the gel’s potential to mimic and surpass the regenerative capacity of the natural hematoma.
Peptide-Blood Gels Enhance Bone Regeneration
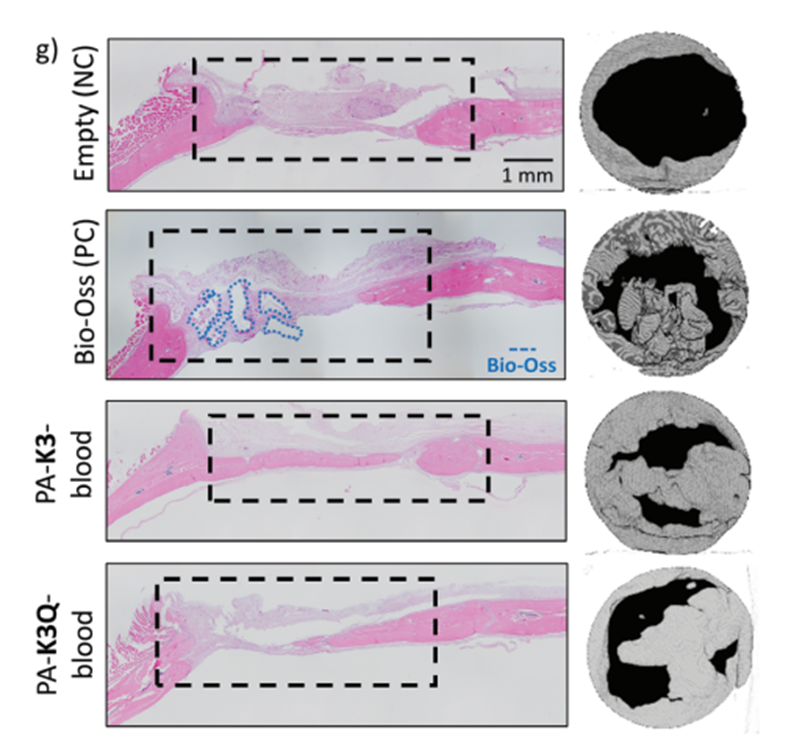
To assess its ability to repair severe injuries, the researchers tested the gel in a critical-sized cranial defect in rats – a bone injury that would not heal on its own. Using each rat’s blood, they created personalized hydrogels and implanted them into the bone defect. After six weeks, the gel significantly improved bone formation, with new tissue filling the defect and resembling healthy bone.
Compared to untreated controls, the gel-treated injuries showed greater bone density and organization. Remarkably, the gel’s performance was comparable to a commercial bone substitute, but with the added advantages of simplicity and personalization. Notably, treated rats exhibited lower levels of inflammatory markers (TNF-α, IL-1β), indicating reduced inflammation and a more favorable environment for healing and bone regeneration. This reduction in inflammation is particularly relevant to elderly patients who often experience chronic low-grade inflammation that can hinder effective healing.
A Breakthrough In Regenerative Medicine
Overall, the study’s findings demonstrate that peptide-blood gels could make a huge impact in the field of regenerative medicine. The personalized nature of the gel, made from a patient’s own blood, minimizes the risk of rejection and makes the technology accessible. Moreover, its adaptability allows for precise 3D printing into customizable shapes and structures, facilitating customized solutions for targeted injuries or specific therapeutic applications.
“The possibility to easily and safely turn people’s blood into highly regenerative implants is really exciting. Blood is practically free and can be easily obtained from patients in relatively high volumes,” explains Dr. Cosimo Ligorio, the study’s co-author. “Our aim is to establish a toolkit that could be easily accessed and used within a clinical setting to rapidly and safely transform patients’ blood into rich, accessible, and tunable regenerative implants.”
Potential Applications in Aging and Longevity
Peptide-blood gels also have potential applications in addressing age-related musculoskeletal disorders, including osteoporosis and arthritis. Their targeted, patient-specific approach could reduce the need for invasive surgical procedures and help maintain functional independence in aging populations.
Moreover, their ability to modulate inflammatory pathways suggests broader applications in geriatric medicine and longevity research. By reducing chronic inflammation associated with aging, peptide-blood gels may slow tissue degeneration and enhance physiological resilience. Their controlled release of growth factors supports regenerative processes, aligning with precision medicine approaches to age-related decline.
By integrating these capabilities, peptide-blood gels represent a scalable and effective solution for addressing both regenerative and age-related medical challenges.
Model: standard critical-sized rat cranial defect model
Dosage: Model 1. PA solution inside blood (or fraction). A small volume of 5 μL
of PA solution (10 mg mL−1) was injected inside a drop of 15 μl of whole
blood or fraction (Plasma/PRP/serum).
b) Model 2. Blood (or fraction) inside peptide amphiphile solution.
A volume of 5 μL of whole blood or fraction (Plasma/PRP/Serum)
were injected inside a drop of 15 μl of peptide amphiphile solution
(10 mg mL−1).

