Revitalizing Female Reproduction: How Spermidine Transforms Aging Oocytes To Increase Fertility
Researchers find that spermidine – a compound naturally found in wheat germ, mushrooms, and broccoli – restores the quality of aged oocytes (female egg cells) and increases fertility in female mice.
Highlights:
- Aged oocytes have lower spermidine levels than young oocytes.
- Supplementing aged 52-week-old mice (similar to a 44-year-old) with spermidine restores spermidine levels and improves the quality of oocytes, leading to increased fertility.
- The beneficial effects of spermidine on oocyte health rely on the activation of mitophagy – the clearing and recycling of defective mitochondria inside a cell.
Reproductive success depends on numerous critical steps unfolding seamlessly, starting from the union of sperm and egg for fertilization to the birth of a child. However, one cannot achieve a healthy pregnancy if their egg cells, also referred to as oocytes, are compromised, an issue that becomes increasingly common with age. In fact, research shows that the chances of getting pregnant within one year are less than 52% for females aged 35 years or older. Thus, scientists have sought to identify novel therapeutics capable of preserving oocyte health.
In a new study published in Nature Aging, researchers from the Nanjing Agricultural University in China report that spermidine supplementation restores oocyte quality and increases fertility in aged female mice. What’s more, the investigators demonstrate that spermidine’s beneficial effects on oocytes are dependent on mitophagy – a cellular process involving the clearance and recycling of defective mitochondria – highlighting a potential target for future oocyte interventions.
Spermidine Restores Oocyte Quality and Increases Fertility
Without a sufficient pool of healthy, viable oocytes, women become increasingly infertile. Oocyte quality is heavily tied to several factors, including genetics, hormone balance, and diet. New evidence suggests that spermidine – a potent anti-inflammatory molecule shown to extend lifespan in model organisms – may also play a role in supporting oocyte quality.
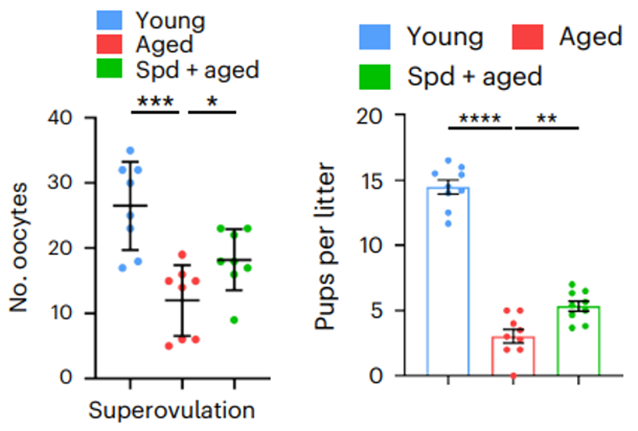
In the present study, Zhang and colleagues examined whether spermidine supplementation could delay ovarian aging in aged female mice, which were found to have reduced spermidine levels, fewer mature oocytes (those ready for fertilization), and impaired fertility. Following 10 days of daily spermidine injections (50 mg/kg/day), aged female mice showed an increase in spermidine levels as well as more mature oocytes, suggesting that spermidine enhanced their oocyte quality.
The investigators then looked at litter sizes to assess spermidine’s effect on fertility. Generally, younger, more fertile mice birth larger litters, and this decreases as the mice age. Predictably, the aged untreated mice had drastically smaller litters than the younger mice. However, aged female mice treated with spermidine gave birth to larger litters, with significantly more pups than untreated mice, further highlighting that spermidine could be an effective means of increasing fertility.
Spermidine Boosts Oocyte Health Through Mitophagy
After establishing spermidine’s role in preserving fertility, researchers wanted to pinpoint the mechanism behind spermidine’s positive impact on oocyte health. Several studies have correlated spermidine’s pro-longevity effects to its ability to activate autophagy – our body’s mechanism for removing cellular waste. Notably, the autophagy of defective mitochondria (fittingly called “mitophagy”) is critical to maintaining functional mitochondria, which contribute heavily to the health and quality of oocytes. With this in mind, the investigators explored whether spermidine’s protective effects stemmed from increased activation of mitophagy.
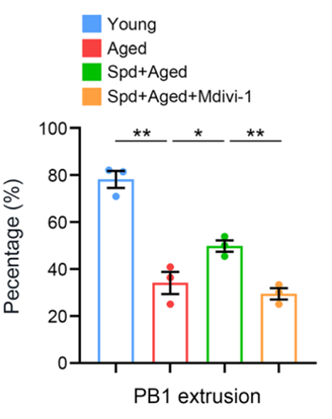
To do so, Zhang and colleagues took oocytes from aged mice and cultured them with spermidine and a mitophagy inhibitor (Mdivi-1) to see if blocking mitophagy blunted spermidine’s effects on oocyte maturation. Oocyte maturation was measured by quantifying the percentage of first polar body (PB1) extrusion, a cellular process indicative of oocyte maturation. The investigators found that the mitophagy inhibitor significantly compromised PB1 extrusion in spermidine-treated aged mice, demonstrating that mitophagy activation is necessary for spermidine to promote oocyte maturation.
Targeting Mitophagy to Promote Longevity
Overall, the study’s findings suggest that the health and quality of aged oocytes can be restored by supplementing with spermidine, the effects of which rely on mitophagy. As previously mentioned, mitophagy activation is paramount to keeping healthy and functional mitochondria. Unfortunately, this critical process progressively deteriorates with age, exacerbating mitochondrial dysfunction. Damaged mitochondria release oxidative stress-inducing compounds – reactive oxygen species (ROS) – as well as trigger chronic inflammation, both of which are hallmarks of aging.
Researchers are continuing to unravel the benefits of maintaining a healthy pool of mitochondria via mitophagy upon aging. What makes this field of study even more compelling is the wealth of mitophagy-inducing compounds that hold anti-aging properties, like nicotinamide mononucleotide (NMN), urolithin A, and fisetin. In light of these findings, it becomes increasingly evident that mitophagy plays a definitive role in preserving overall health and delaying the onset of critical age-related characteristics.
Model: Young (~6–8-week-old) and aged (~52–56-week-old) ICR female mice.
Dosage: Aged mice were intraperitoneally injected daily with spermidine (Sigma-Aldrich; 50 mg per kg body weight per d, dissolved in PBS) or the equivalent volume of PBS.

