Rejuvenation: The Science of Reversing Biological Age
Several interventions have recently emerged to reverse rather than just attenuate aging.
There’s a lot of talk these days about stopping or slowing down aging. But what about rejuvenation — the reversal of aging? Can we actually turn back the clock to become biologically younger?
Anti-aging vs. Rejuvenation
First things first: what constitutes rejuvenation, and how does it differ from anti-aging?
The line between rejuvenation therapies and other longevity interventions, such as those that slow down or prevent aging, is hazy, and these strategies are frequently used interchangeably. Anti-aging indicates the maintenance or preservation of aging biomarker status. This is typically measured by biomarkers of aging, such as clocks based on DNA modification patterns or the length of telomeres — the protective DNA caps of chromosomes.
Rejuvenation goes one step further and requires a strong, prolonged, and systemic reduction in biological age. A more important feature common to the existing and potential rejuvenation interventions is the exceptional enhancement of regenerative capacity.
The reversal of aging is inherently multidimensional: it may include a reduction in damage at the molecular level, renewed cell functionality at the cellular level, and meaningful physiological improvement at the organismal level. Some age reversal therapies may also induce lifespan extension. Along these lines, the effect at one level of biological organization is usually accompanied by connected effects at other levels.
Rejuvenation Strategies
Rejuvenation strategies have much in common with existing longevity interventions. Although most longevity interventions cannot systemically reverse biological age like rejuvenation therapies, they attenuate certain age-related hallmarks, with essential effects such as the reduced presence of senescent cells and increased stem cell pool size and functionality.
Some rejuvenation strategies have been shown to reverse epigenetic age, increase stem cell function, reverse age-related loss of eyesight, and increase the lifespan of mouse models. Interventions usually influence age-related characteristics across multiple levels, and robust measures (biomarkers) of age-related damage at one level could be used to identify putative rejuvenation interventions.
There are all sorts of strategies for rejuvenation, ranging from the relatively innocuous like exercise and taking supplements to the more “daring” like gene therapy and organ transplantation.
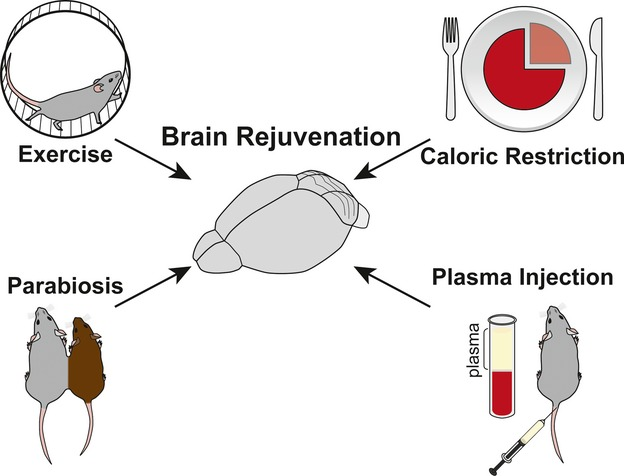
Exercise & Other Physiological Regimens
Physical exercise increases blood delivery to most tissues and leads to changes in the systemic environment. Interestingly, numerous studies have documented rejuvenating effects of exercise on the functional and regenerative capacity of peripheral tissues and central nervous system (CNS) in animal models. Outside the CNS, exercise can promote hematopoiesis (regeneration of blood cells) in the aging systemic environment, and increase the proliferative capacity of aged skeletal muscle stem cells. There is research showing that exercise can enhance telomerase activity to protect and even extend telomere length.
For people who cannot exercise, there are other activities that can influence systemic rejuvenation. For example, another study showed that hyperbaric oxygen therapy – exposure to 100% oxygen at elevated environmental pressure to optimize body tissue oxygen absorption – extends telomeres 20-38% in different immune cell types and reduced the senescent cell population 11-37%, depending on the type of cell.
Caloric restriction
Another systemic manipulation shown to counteract the age-induced effects on tissue regeneration is caloric restriction, a reduction of 20–40% of caloric intake without malnutrition. Caloric restriction has been shown to rejuvenate tissue regeneration in aged organisms, similar to the effects of exercise. A number of studies have shown rejuvenating effects of caloric restriction on the decline of hematopoietic stem cell function. Rejuvenation of regeneration was also observed in skeletal muscle and on intestinal stem cells. The effects of both short-term and long-term caloric restriction on rejuvenation of regeneration are also found in the central nervous system – the brain and spinal cord.
NAD+ Homeostasis
Supplementation through NAD+ precursors in specific settings may also reverse hallmarks of aging, such as improving telomere length. For example, Increasing NAD+ levels has been shown to not only protect but also improve telomere length. One study in mice showed that the NAD+ precursor nicotinamide mononucleotide (NMN) treatment improved the length of telomeres in mice.
Senolytics
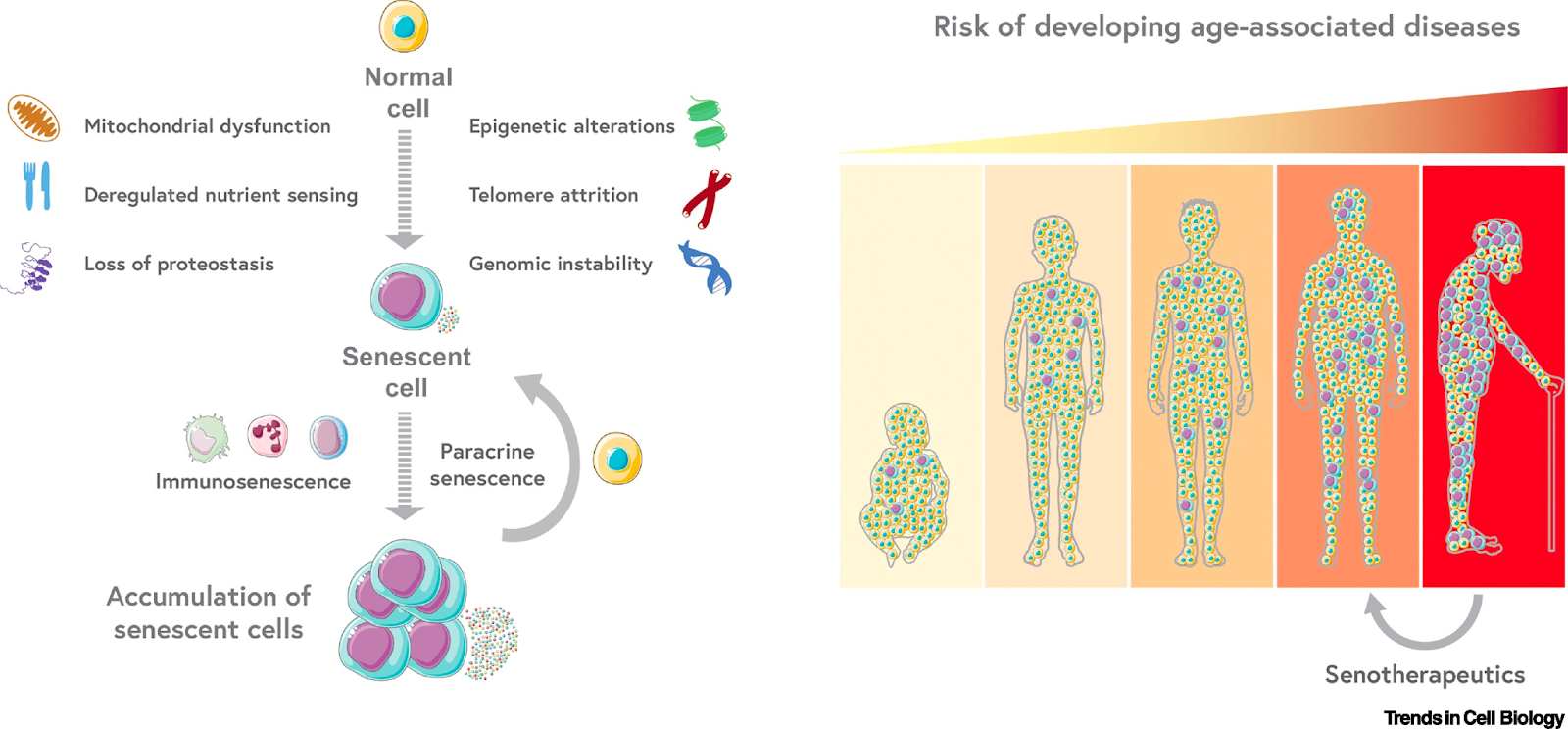
There is increasing evidence of the detrimental role of senescent cells in aging. Some of the hallmarks of aging (mitochondrial dysfunction, deregulated nutrient-sensing, loss of proteostasis, epigenetic alterations, telomere attrition, and genomic instability) induce normal cells to become senescent, which in turn can induce paracrine senescence in nearby normal cells through senescence-associated secretory phenotype (SASP). Senescence-promotion through SASP together with a decline in the immune system activity, converge to induce organismal accumulation of senescent cells.
In aged individuals, chronic accumulation of senescent cells contributes to tissue dysfunction and increased risk of age-associated diseases development. Clearance of senescent cells has improved age-associated pathologies in animal models, leading to promising new clinical trials. Different mechanisms of senescent cells can be exploited pharmacologically to develop new therapeutic targets. Senescent cells elimination with different senotherapeutic approaches can improve healthspan in aged individuals.
Telomere Extension
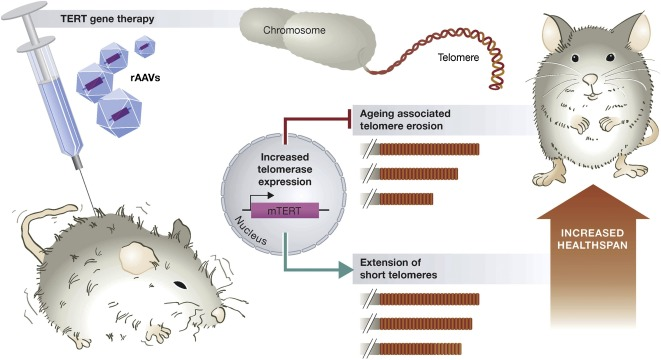
Aging is linked to shortening telomeres. This happens, in part, because of insufficient activity of an enzyme called telomerase reverse transcriptase (TERT) that maintains telomere length. Animals deficient in TERT have shorter telomeres, shorter lifespans, and an increased risk of age-related diseases like heart disease. Recent studies on animal models have shown the therapeutic efficacy of TERT in increasing healthy longevity and reversing the aging process.
Telomeres can be extended in various genetic, pharmacological, and physiological means, typically by activating TERT. For example, one screen identified small molecules that activate TERT and extend telomeres.
Another major route to elongate telomeres has been through gene therapy with TERT, either by injection or even intranasal administration. For example, a study that used safe and effective viral TERT gene therapy strategies increased telomere length in the heart, liver, kidney, brain, lung, and muscle in 2-year-old-mice by six times than in control mice of the same age. Notably, the mice treated with TERT gene therapy demonstrated healthier aging and extended longevity.
Cellular reprogramming
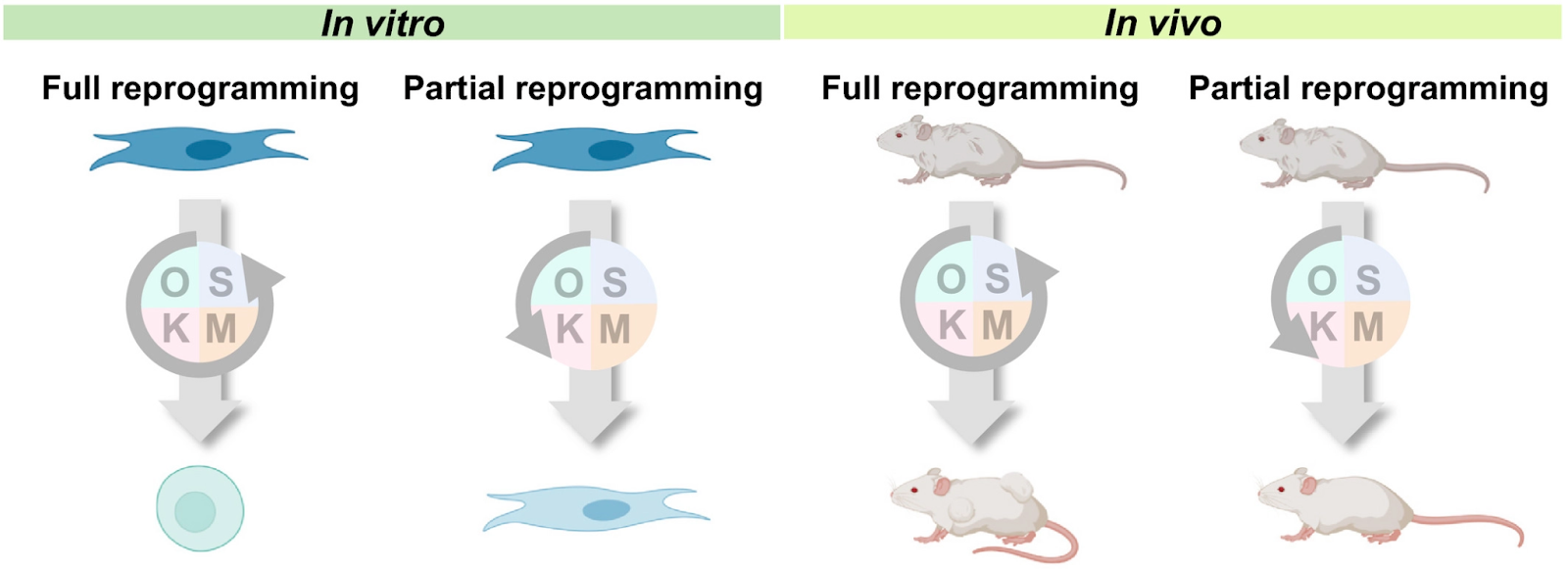
On a microscopic scale, the most extreme case of heterochronic transplantation is somatic cell nuclear transfer, which has emerged as the basis for modern-day cloning approaches (Gurdon et al., 1958). By transferring an adult cell nucleus to a de-nucleated oocyte, a new individual can be generated. This technique encapsulates the full potential of reversing the biological age of a somatic cell to that of the new embryo. Interestingly, this implies that methylation patterns in the transferred nucleus are likely to reset by cytosolic components in the oocyte, suggesting another potential mechanism for rejuvenation.
To recapitulate this effect without the introduction of complex microscopic procedures, scientists discovered four critical “reprogramming factors” (Yamanaka factors), which, when expressed in somatic cells, could effectively reverse the developmental status to that of early embryos, generating induced pluripotent stem cells (iPSCs). Interestingly, low epigenetic ages around zero were predicted when epigenetic clocks were applied to iPSC samples. Almost all clocks showed considerable epigenetic age decreases compared with dermal fibroblasts used as the source of fully reprogrammed iPSCs.
Similar characteristics were observed in mice. In the case of mice, clocks reported a range of epigenetic ages of the identical iPSCs. Taken together, most human and mouse clocks reach a consensus in establishing age reversal that occurs as a result of reprogramming, although consistent predictions of age in these cells across different models remain a challenge.
Heterochronic transplantation
Since the 1960s, and perhaps even earlier, researchers have been transplanting tissues and organs from animals of one age to animals of different ages. These “heterochronic” age chimeras have shown rejuvenating properties.
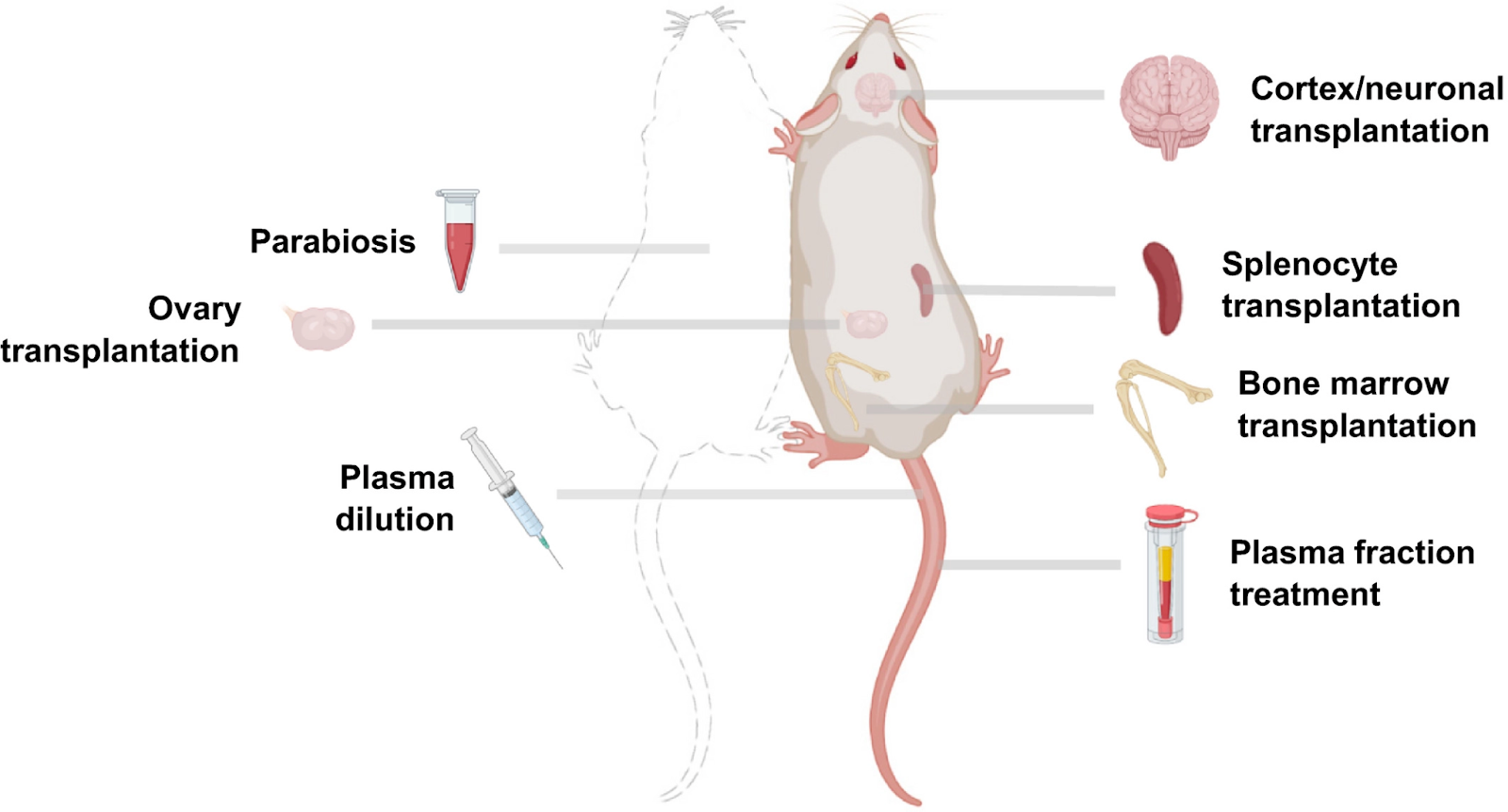
Of the potential rejuvenation therapies that remain to be thoroughly characterized, linking the circulatory systems between a young and old organism – heterochronic parabiosis – is one of the most notable. This surgical procedure has been performed for years on rodents, and it was shown that mouse lifespan could be extended by linking the circulatory system of an old mouse with that of a young mouse. Heterochronic parabiosis was rediscovered as one of the most promising rejuvenation interventions in 2005. By briefly connecting the circulatory system of young and aged mice, old mice exhibited youthful features in the brain, muscle, and liver, characterized by increased cognitive function, replenished stem cell pools, and augmented regenerative capacity.
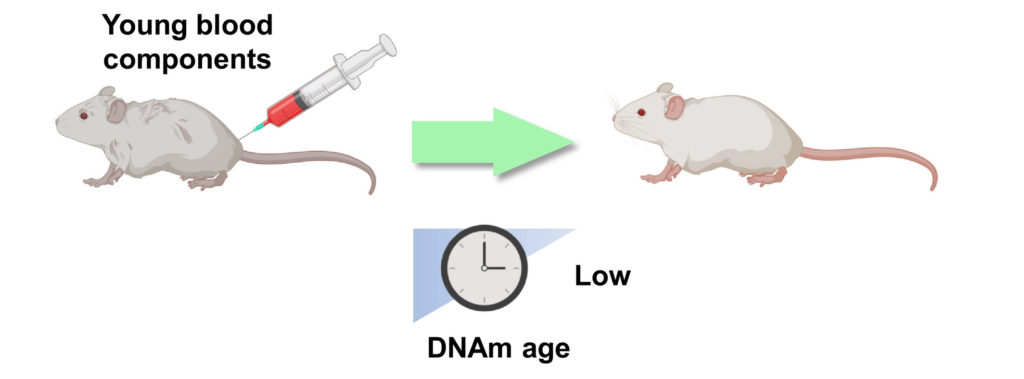
Following up on this study, researchers have focused on blood components for biological age reversal, while others have investigated the transplantation of other organs to replace aged tissues. With bone marrow transplantation, the blood epigenetic age of the recipients generally matches the age of the donors, although whether this effect is systemic has yet to be established. Of note, bone marrow transplantation has also shown a 12% increase in mouse lifespan.
Additionally, a study using an undisclosed plasma fraction demonstrated robust reversal of epigenetic age, further suggesting that heterochronic transplantation may be a potential rejuvenation intervention. Recent work has also revealed that young splenocyte transplantation ameliorates the aging features of progeroid animals. In addition, transplantation of embryonic brain tissues has shown potential for neuronal repair, and ovarian transplantation was reported to improve health parameters and result in an extended lifespan.
The Future of Rejuvenation
Overall, rejuvenation procedures provide promising prospects to reverse people’s biological ages, thereby prolonging life and improving health. However, many of the biological underpinnings of rejuvenation remain unknown, and the present adverse effects generated by some of these therapies (especially reprogramming) prohibit their widespread implementation. Existing methods, such as lowering the dose and regularly monitoring key statistics, can assist to mitigate these side effects.
However, in the future, extensive multi-modal assessments of the common aspects of present and emerging rejuvenation therapies will be important to remove these detrimental consequences. It will eventually be feasible to assess and harness the underlying links between age-reversing techniques as more of these therapies are created and independently validated based on molecular and physiological aging biomarkers. Finally, our work at the intersection of aging, molecular profiling, high-resolution methods, and physiological assessments may pave the way for the safe and successful use of systemic rejuvenation medicines in people.

