Researchers Alleviate Diabetes By Deleting Senescent Fat Cells
UConn Health and Mayo Clinic study shows that drugs that eliminate senescent cells in human fat ease signs of diabetes
Highlights
· Specific senescent cells accumulate in fat with obesity.
· Intermittent clearance of these senescent cells both prevents and alleviates insulin resistance.
· A drug combination of dasatinib and quercetin reduces these senescent cells in human fat and alleviates metabolic harm in rodents.
More than 93 million US adults are obese. The condition of having too much body fat accelerates the aging processes and is among the most significant risk factors for the development of type 2 diabetes, one of the leading causes of death for older adults. Insulin resistance — impaired tissue responses to insulin signaling that results in abnormal insulin secretion — is the hallmark and the earliest detectable abnormality for prediabetes, a precursor of type 2 diabetes. But besides lifestyle changes, such as exercise and a healthy diet, therapeutic targets for insulin resistance are lacking.
Researchers from UConn Health and the Mayo Clinic show that eliminating specific senescent cells, in fat tissue alleviates insulin resistance in obese mice. Importantly, removing these senescent cells, which have high activity in the levels of a gene called p21, in human fat tissue mitigates insulin resistance when transplanted into mice. These findings lay the foundation for targeting senescent cells as a new therapy to alleviate insulin resistance and impede the development of type 2 diabetes.
“These drugs can make human fat healthy, and that could be great,” says senior author Dr. Ming Xu. “The results were very impressive and cleared the route for potential clinical trials.”
Targeting senescent cells in fat tissue alleviates insulin resistance in obesity
There is some evidence demonstrating that senescent cells have tissue-specific roles in animals and humans. This is important to note because senolytics are not typically administrated to specific tissues; they’re typically administered systematically. But, when they are administered targeting a specific tissue, senolytics can have beneficial effects. For example, one study indicated that clearance of certain sensecent cells in the pancreas, in this case ones that had high activity of a gene called p16, contributed to improved glucose tolerance. However, such studies cannot exclude the contribution from other tissues, such as the liver, fat tissue, and muscle.
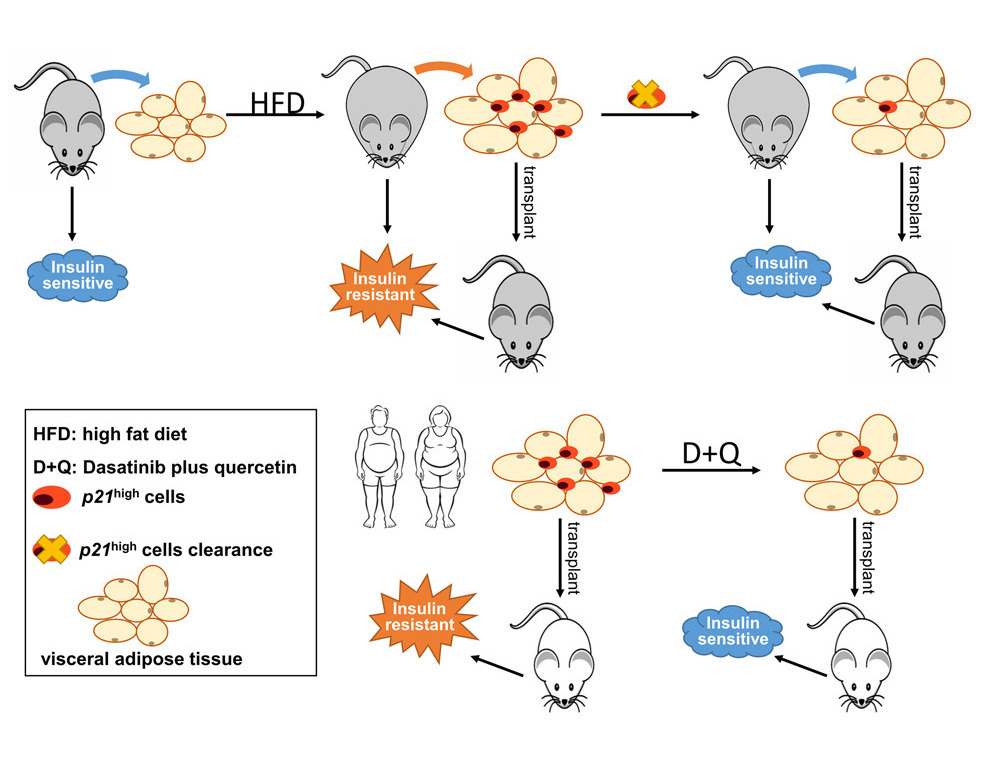
In this study, Wu and colleagues provide evidence that clearance of cells with high activity of the p21 gene (p21high cells) only in fat tissue is sufficient to prevent obesity-induced metabolic dysfunction. Intermittent clearance of p21high cells alleviates metabolic dysfunction and has long-term benefits protecting against metabolic dysfunction in obese mice.
Wang and colleagues say that their study implicates cells with high activity in the p21 gene are a new and potentially translatable target for type 2 diabetes interventions. Along these lines, they show that monthly clearance of p21high cells can provide long-term protective effects on insulin resistance in obese mice. Notably, at two of the time points during p21high cells clearance (two months and four months after being fed a high-fat diet), the metabolic benefits are comparable, which further enhances the translational potential of this approach.
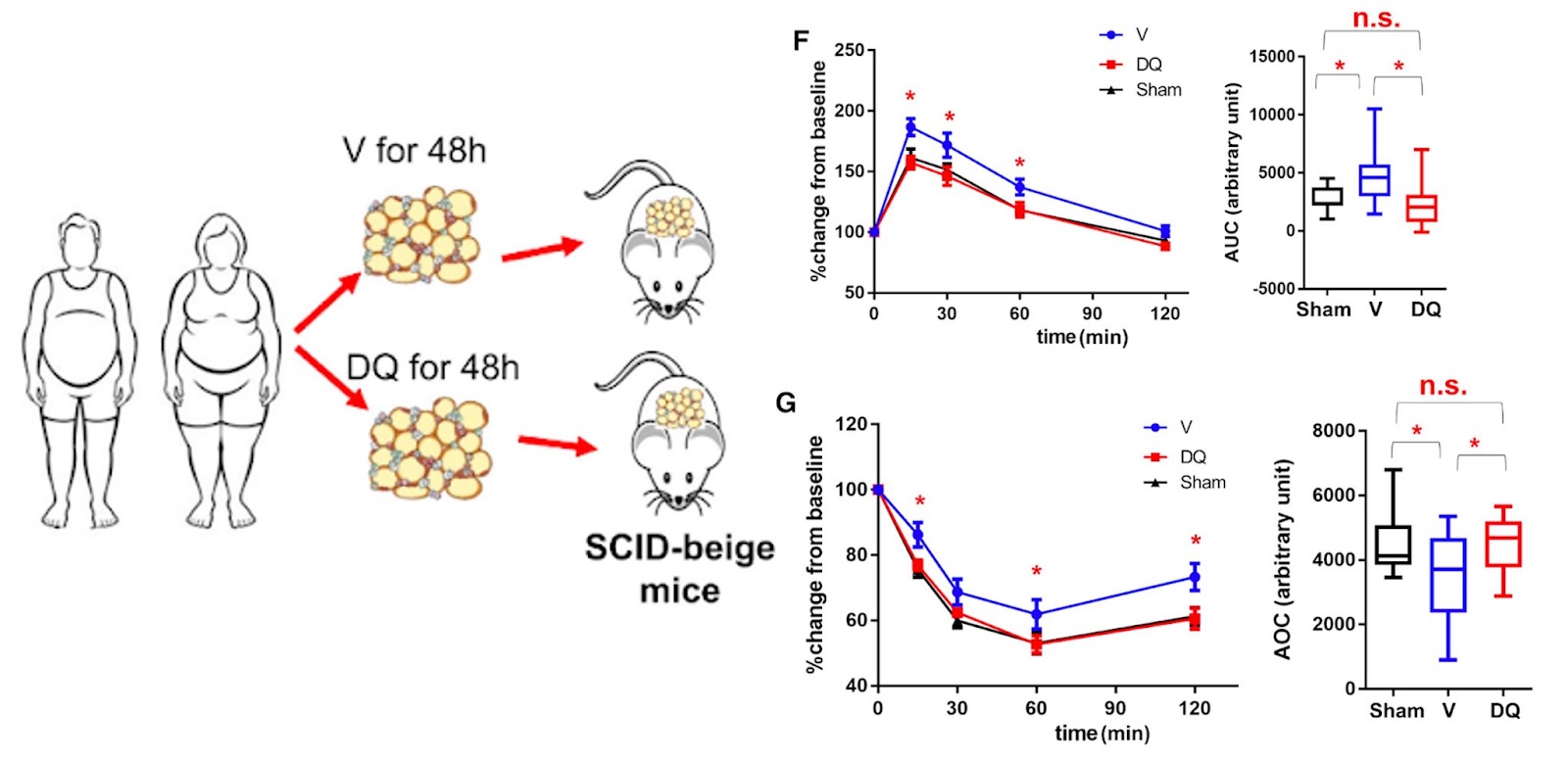
To support these claims, Wu and colleagues show that p21high cells can be eliminated by the senolytic cocktail of dasatinib and quercetin in human fat tissue to support these claims. When these human fat cells are then transplanted into mice, the mice don’t experience the same metabolic dysfunction observed in mice that received untreated cells. In fact, the metabolism of these mice, as it pertains to glucose and insulin, returns to the levels observed in mice that didn’t receive any human fat cells at all.
Targeting of senescent cells as a new therapy to prevent diabetes
This could represent essential knowledge for developing fat-specific interventions to treat metabolic dysfunction while minimizing the potential undesirable side effects from clearance of senescent cells in other tissues. The findings here fill an important knowledge gap by demonstrating the benefits of dasatinib plus quercetin on human fat tissues and provide support for clinical trials testing dasatinib plus quercetin in insulin resistance and type 2 diabetes.
Of note, clearance of p21high cells is completely distinct from interfering with p21 gene activity and function. The p21 gene plays an essential role in many cellular events, and inactivation or suppression of the p21 gene can lead to tumor formation. The strategy here is to eliminate a small portion (2%–15%) of cells that highly express p21 while not significantly reducing p21 gene activity in other cells. This is supported by the fact that the clearance of p21high cells results in only roughly a 25% reduction of p21 activity in the whole population of non-fat cells found in fat tissue.
In addition, Wang and colleagues show that p21high cells can be pharmacologically eliminated by dasatinib plus quercetin in human fat tissue. In these mice, Wang and colleagues performed glucose and insulin tolerance tests, which are common lab tests that check how your body moves sugar from the blood into tissues like muscle and fat and how insulin is produced in response. Mice that received human fat treated with dasatinib and quercetin had decreased glucose and increased insulin tolerance test scores than those that received untreated human fat levels and became comparable to those seen in mice that did not receive human fat.
Recently, intermittent administration of dasatinib plus quercetin has proven relatively safe in older human individuals and can effectively reduce p21high cells in adipose tissue in human participants. The findings here fill an important knowledge gap by demonstrating the benefits of dasatinib plus quercetin on human fat tissues and provide support for clinical trials testing this senolytic combination in insulin resistance and type 2 diabetes.
It makes sense then that, in a press release, Xu said, along with his colleagues at UConn and the Mayo Clinic, he is now pursuing using the dasatinib and quercetin combination in clinical trials to see if the drugs can improve Type 2 diabetes in human patients.
“Although these preclinical results were very promising, large-scale clinical trials are absolutely critical to examine the efficacy and safety of these drugs in humans before clinical use,” says Dr. Xu. Moreover, the human adipose tissue transplantation model developed here can be leveraged to screen a range of senolytic drugs or other agents on human individuals’ fat tissues to alleviate insulin resistance, which has the potential to be an invaluable tool for future precision medicine.

