Removing “Zombie” Cells With Senolytics Improves Bone Fracture Healing in Young Mice
Mayo Clinic researchers cleared non-proliferating (senescent) cells with senolytic combo to accelerate fracture healing.
Highlights
· Saul and colleagues find non-replicating (senescent) cells accumulated during bone healing in young adult mice.
· Following a fracture, intermittent treatment with compounds that selectively eliminate senescent cells (senolytics) heals bones twice as fast.
· The resulting bone had an increased volume and improved biomechanical properties, such as increased stability.
Our bones become brittle with aging, making them susceptible to cracks, breaks, and deterioration. As with other tissues, non-dividing (senescent) cells accumulate within the aged bone and cause age-related bone loss. Curiously, though senescent cells have detrimental effects across tissues with aging, they appear to have a role in tissue repair. So far, researchers have found that senescent cells play critical roles in skin wound healing, but the potential role of senescent cells in fracture healing is undefined.
Mayo Clinic researchers published research in Elife showing that treating young adult mice following fracture with drugs that selectively kill senescent cells called senolytics speeds up bone healing. When using the senolytic combination of Dasatinib plus Quercetin, Saul and colleagues could halt the senescence-driven cascade to improve fracture healing in mice.
“Collectively, our findings have clinical implications for the development of senolytic therapies for osteoporosis and also have biological relevance for our concept of senescent cells as facilitating healing, as these beneficial versus detrimental effects of senescent cells on injury repair may vary across tissues,” concluded Saul and colleagues.
Senescence and Tissue Repair
Although senescent cells are usually associated with aging, in youth, beneficial functions of these non-dividing cells have been suggested in skin wound healing. In the cutaneous setting, senescent cells attract various cell populations like immune cells to accelerate skin wound healing. However, the dynamics of skin wound healing do not directly translate to the different bone healing repair phases. Also, bone has the exclusive ability to form scar-free tissue. Nevertheless, whether senescent cells show up in the healing skeleton or how these cells modulate fracture repair is unknown.
Senescent Cells Appear at Bone Breaks
To avoid the confounding effects of senescence with aging, Saul and colleagues specifically focused on fracture healing in young adult mice. Using mice genetically altered animals to mark senescent cells, the Mayo Clinic researchers found that senescent cells appear at the bone callus — the repair site following a bone fracture. Interestingly, when they deleted the senescent cells, the process of bone formation after the fracture sped up. These findings point to senescent cells hurting bone repair, in contrast to their beneficial effects in skin wound healing.
Senolytics Improve and Accelerate Fracture Healing
Saul and colleagues then treated young adult fractured mice with senolytics to evaluate whether clearance of senescent cells adversely affected fracture healing, which is a critical issue in further developing senolytic therapies for osteoporosis. Studies have shown that intermittent delivery of the senolytic cocktail, Dasatinib (a tyrosine kinase inhibitor) plus Quercetin (a natural flavonoid), eliminates senescent cells in old mice in various tissues. In human and preclinical studies, Dasatinib plus Quercetin slows the onset of aging by preventing multiple age-related ailments, extending healthspan. These findings are now being translated into humans, and Dasatinib plus Quercetin is currently undergoing human trials for safety and efficacy to prevent age-related skeletal deterioration (NCT04313634).
To test whether Dasatibin plus Quercetin can rescue senescence in bone marrow stem cells, the Mayo Clinic researchers isolated, cultured, and treated mouse bone marrow stem cells, which give rise to newly forming bone, with the senolytic cocktail. Compared to untreated bone marrow stem cells, the senolytic cocktail had minimal but negligible effects on the total number of cells in culture. However, when these cells were induced to become senescent, Dasatinib plus Quercetin had a major impact on preventing the buildup of senescent cells.
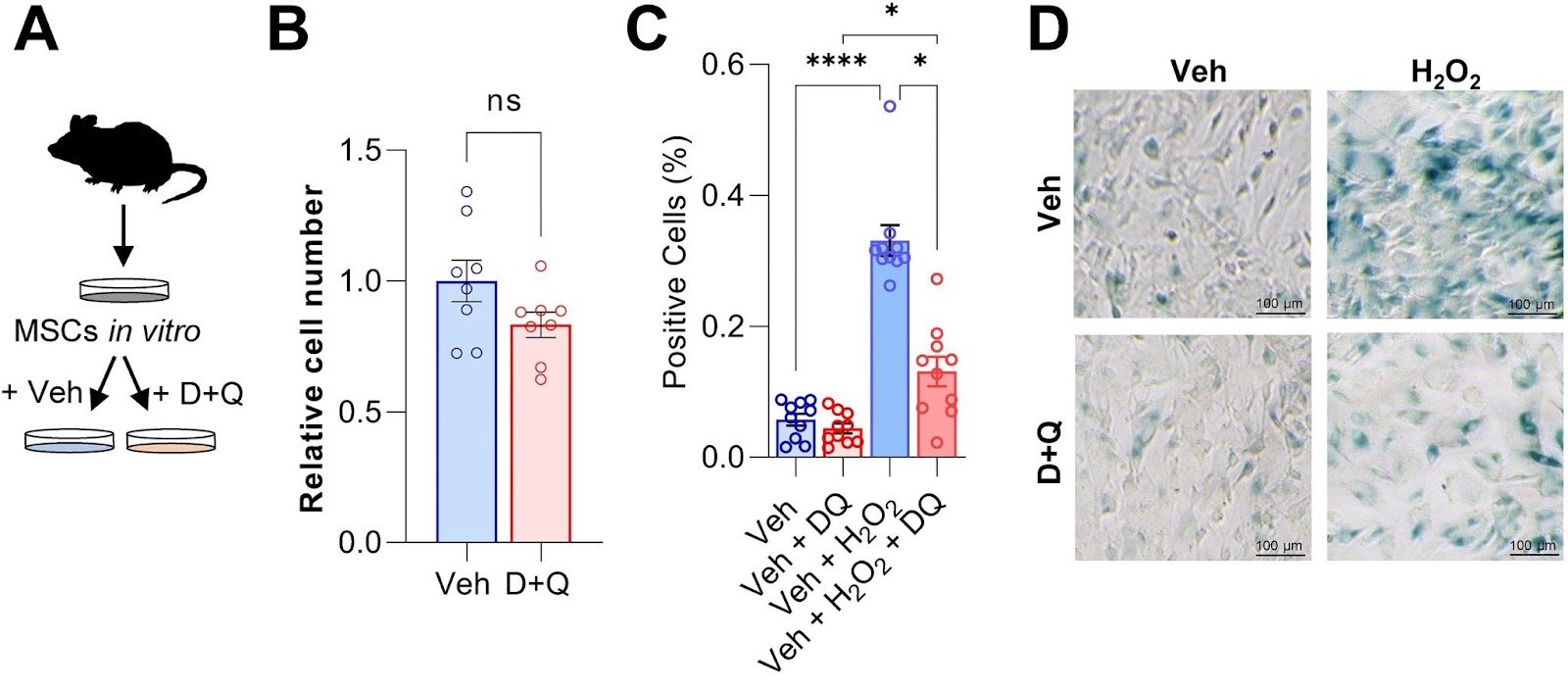
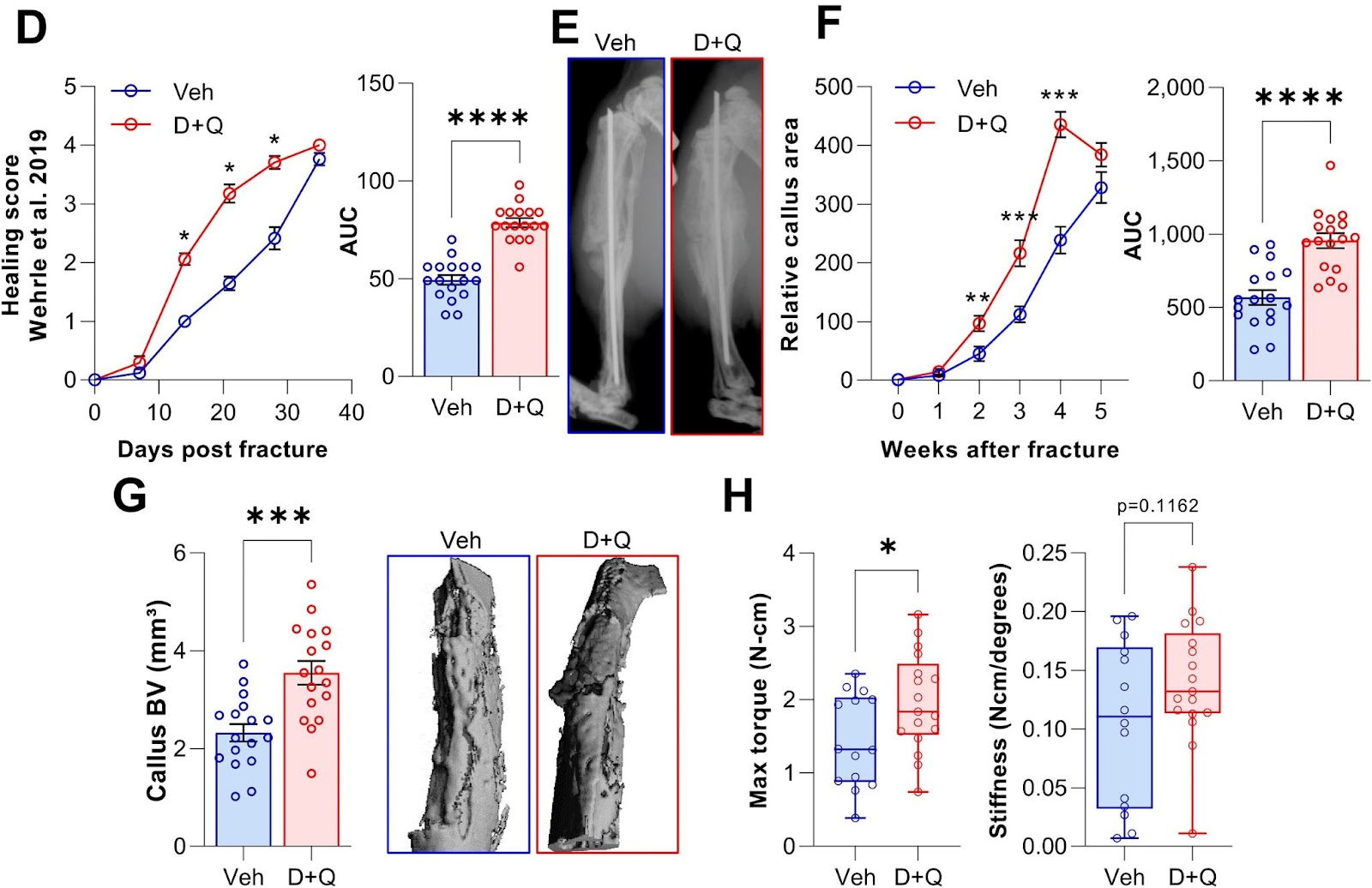
To test if clearance of senescent cells using senolytics would enhance fracture healing, Saul and colleagues treated young mice with Dasatinib plus Quercetin one day before fracture and weekly post-fracture. The Mayo Clinic researchers observed an accelerated healing time after the clearance of senescent cells with the senolytic combo, with greater bone volume after a fracture. What’s more, senolytic treatment resulted in a significant increase in the maximal torque — the resistance to breaking — of the healed bones that could sustain before fracture. Thus, Dasatinib plus Quercetin treatment improved and accelerated the time course of fracture healing.
“Our data demonstrate that this reduction is sufficient to enhance callus formation and at least does not impair the biomechanical properties of the healed bone,” propose Saul and colleagues.
Can Senolytics Repair Bone in People?
In addition to implications for senolytic therapies for osteoporosis, Saul and colleagues say that these findings also have potential biological importance. Specifically, the concept of senescent cells as physiologically facilitating tissue repair — which, to date, has been definitively demonstrated principally in the skin — may be overly simplistic. Thus, contrary to the skin, bone can recover without losing its integrity and forming a scar fully.
As such, the brief appearance of senescent cells may be an evolutionarily conserved mechanism during injury repair, with beneficial effects on skin wound healing, which is associated with scar formation. By contrast, the lack of scar formation in bone and the already highly inflammatory state following fracture may render these transiently appearing senescent cells less helpful and, as demonstrated in this study, potentially detrimental to injury repair in bone.
“Clearly, further studies are needed to test this hypothesis, but our findings should stimulate a reconsideration of senescent cells as generally beneficial in the setting of tissue injury and repair,” concluded Saul and colleagues.

