Pro-Longevity Molecules in ‘Young Blood’ Rejuvenate Aged Mouse Muscle
Researchers identify a crucial mediator of youthfulness for mouse muscle in membranous particles circulating the bloodstream, a discovery that could advance muscle regeneration therapies for older people.
Highlights
- Blood from young mice rejuvenates aged muscle through membrane-bound packages in the blood called extracellular vesicles (EVs).
- Aging affects the cargo carried by EVs, reducing mRNA levels that encode a pro-longevity protein called Klotho.
- Injection of EVs containing Klotho mRNA improved muscle regeneration, copying the effects of blood from young mice on aged muscle.
From some freaky Frankenstein-like studies where researchers sowed together the blood vessels of young and old mice, allowing blood to exchange between the two rodents, researchers showed that circulating factors play a critical role in regeneration and rejuvenation. Beyond carrying oxygen, nutrients, and hormones to cells while removing waste products, like carbon dioxide, blood carries factors that affect the aging and function of stem cells and tissues, including muscle. While many of these factors have been identified as freely circulating proteins, studies have shown that there are membranous particles secreted from cells called extracellular vesicles (EVs), which traffic between anatomically remote sites and serve as biomolecule couriers.
In a study published in Nature Aging, University of Pittsburgh Medical Center (UPMC) researchers demonstrate that EVs carrying particular genetic material drive the beneficial effect of blood from young mice on aged muscle regeneration. Sahu and colleagues show that EVs containing mRNA encoding the pro-longevity protein α-Klotho (Klotho) can mimic the effects of blood from young mice on aged skeletal muscle.
“We’re really excited about this research for a couple of reasons,” said senior author Fabrisia Ambrosio, Ph.D., director of rehabilitation for UPMC International and associate professor of physical medicine and rehabilitation at Pitt. “In one way, it helps us understand the basic biology of how muscle regeneration works and how it fails to work as we age. Then, taking that information to the next step, we can think about using extracellular vesicles as therapeutics to counteract these age-related defects.”
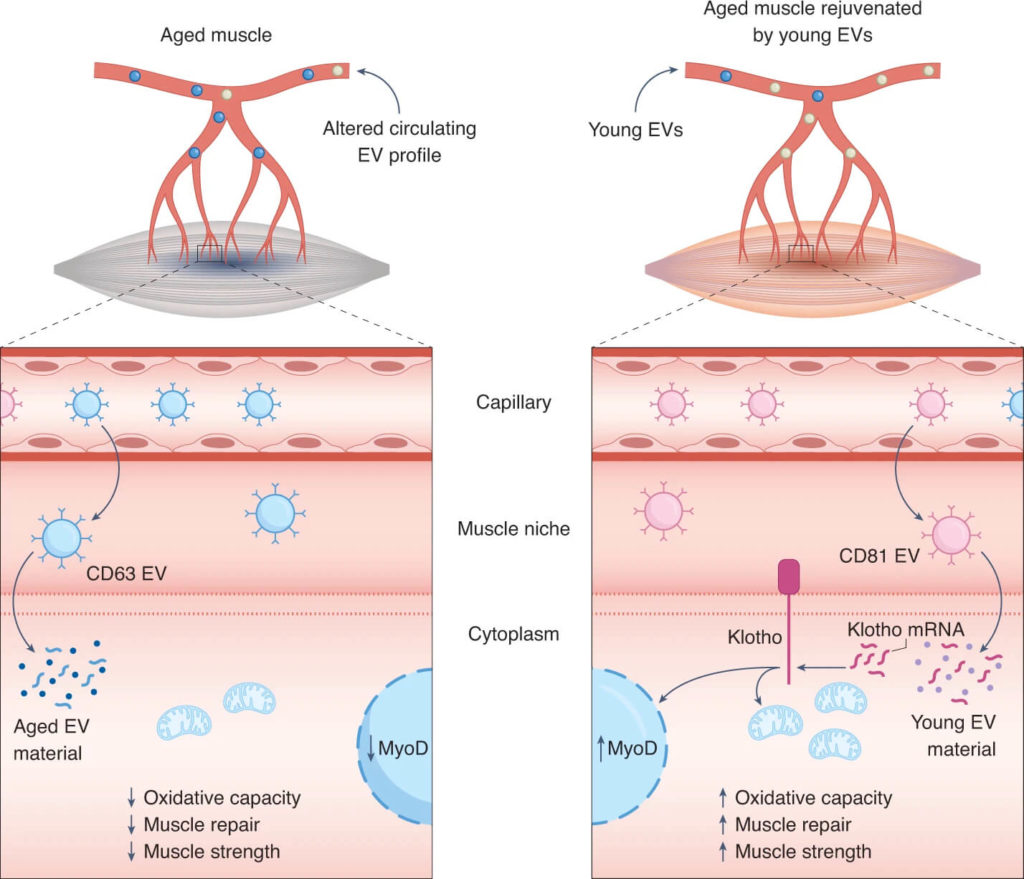
(Ancel and Feige 2021 | Nature Aging) Young EVs rejuvenate skeletal muscle regeneration during aging through the shuttling of Klotho transcripts. With age, skeletal muscle loses its potential to regenerate after tissue damage. Sahu et al. report that aging alters the pool of circulating EVs by skewing their profile toward CD63-expressing EVs that lack mRNA of the anti-aging factor Klotho. Left, these changes compromise downstream benefits of EVs on commitment and mitochondrial oxidative metabolism of myogenic cells, resulting in impaired muscle repair and reduced strength. Right, conversely, injection of young EVs with high expression of CD81 into aged recipients shuttles Klotho transcripts back to muscle cells and improves muscle oxidative capacity, regeneration, and strength.
Aged muscle rejuvenated by exposure to blood from young mice
Circulating factors play crucial roles in regulating tissue balance and regeneration. One approach used to identify the effects of these circulating factors on target tissues involves the surgical joining of a young and old mouse. This procedure enables heterochronic blood exchange — the coupling of circulatory systems from two animals, which exposes the aged mouse to circulatory factors from the young mouse and vice versa. This model has provided valuable insight into the influence of age-associated factors on the function of stem cells and tissues, including skeletal muscle.
Most studies investigating the beneficial effect of heterochronic parabiosis on aged tissue function have primarily focused on free circulating proteins. But a growing number of findings have demonstrated that many biomolecules secreted by cells are packaged within EVs, which traffic between anatomically remote sites and serve as couriers of proteins and genetic material. EVs have been identified in most bodily fluids, including plasma, serum, urine, saliva, and cerebrospinal fluid. Emerging evidence suggests that mRNA cargoes within EVs can target and reprogram cells in various tissues to regulate function.
Given their potent role in intercellular communication, it is not surprising that age-related alterations in the EVs and their cargo have been increasingly associated with age-related diseases. However, whether circulating EVs mediate the ‘rejuvenating’ effects of young blood on skeletal muscle regeneration in old mice has not been investigated. “We wondered if extracellular vesicles might contribute to muscle regeneration because these couriers travel between cells via the blood and other bodily fluids,” said lead author Amrita Sahu, Ph.D., a postdoctoral fellow in the Department of Physical Medicine and Rehabilitation at Pitt. “Like a message in a bottle, EVs deliver information to target cells.”
Circulating extracellular vesicles regulate regeneration of aged skeletal muscle
In this study, Sahu and colleagues evaluated the contribution of circulating EVs to the beneficial effect of young serum on aged muscle stem cells and the skeletal muscle regenerative cascade. The UPMC researchers showed that young serum restores a youthful bioenergetic and myogenic profile to aged muscle cell progeny from muscle stem cells. This effect depends on the presence of circulating EVs. Moreover, whereas youthful EV cargoes preserve mitochondrial structure and function in target muscle cells, aged EV cargoes propagate mitochondrial dysfunction.
Sahu and colleagues next evaluated whether circulating EVs contribute to the previously reported beneficial effects of young serum on skeletal muscle regeneration in mice. For this, aged mice received serial tail-vein injections of young serum as is or depleted of EVs. By 11 days after injury, blood exchange significantly enhanced skeletal muscle regeneration and functional recovery, as determined by myofiber cross-sectional area and contractile testing, respectively. However, the beneficial effect of young serum on the regenerative potential of muscle was lost in the absence of circulating EVs. This shows that the beneficial effect of young serum on aged muscle regeneration and function is dependent, at least in part, on circulating EVs.
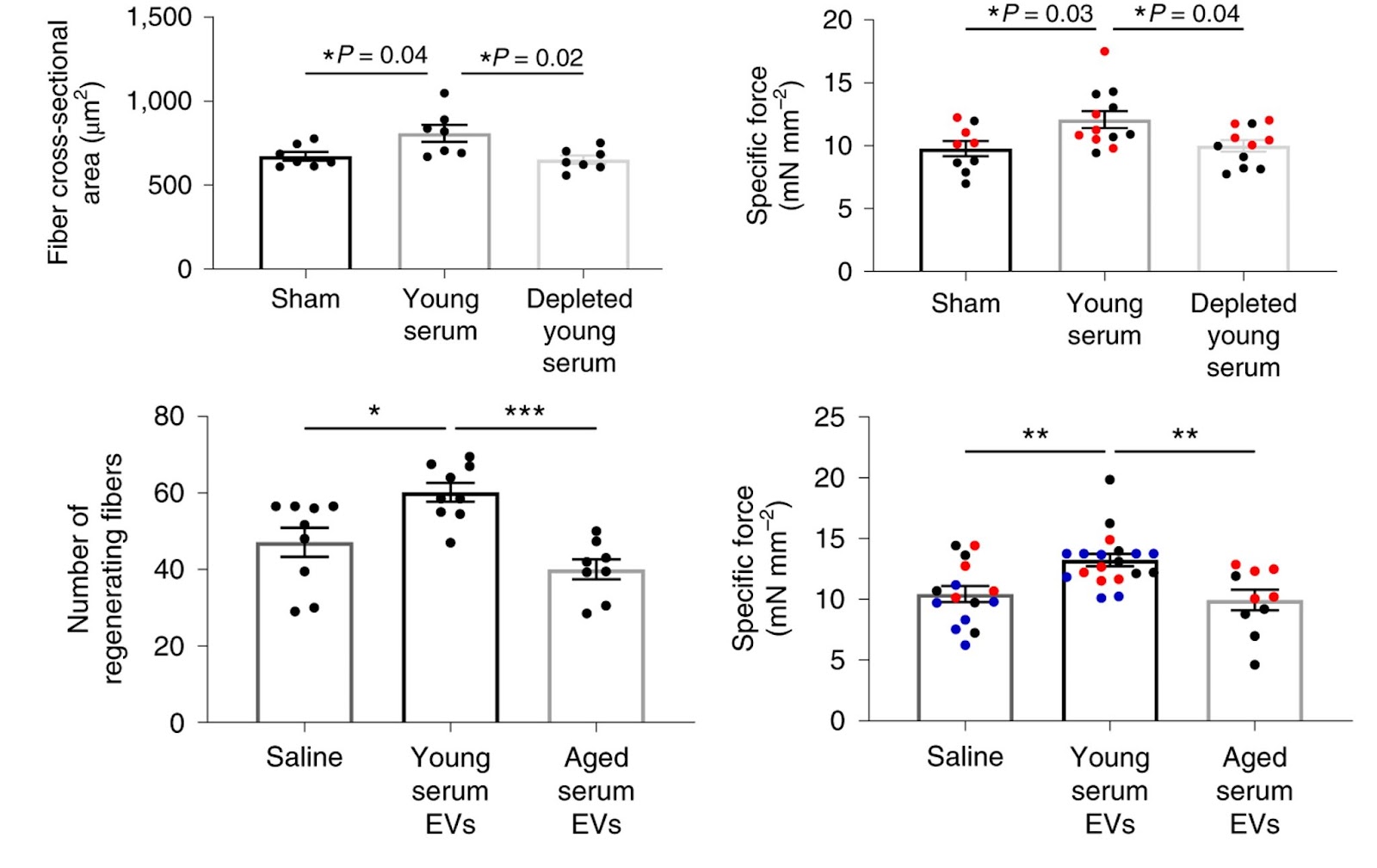
Klotho mRNA within EVs contributes to functional skeletal muscle regeneration
To understand what was driving the beneficial effect of young serum, Sahu and colleagues cataloged the EV cargo. They found that EVs display an age-associated decrease in mRNA levels of Klotho. This longevity protein has been positively associated with maintaining mitochondrial bioenergetics and skeletal muscle health. Notably, the researchers found that the impact of young EVs on muscle progenitor cell bioenergetics and skeletal muscle regeneration depended on the presence of Klotho mRNA.
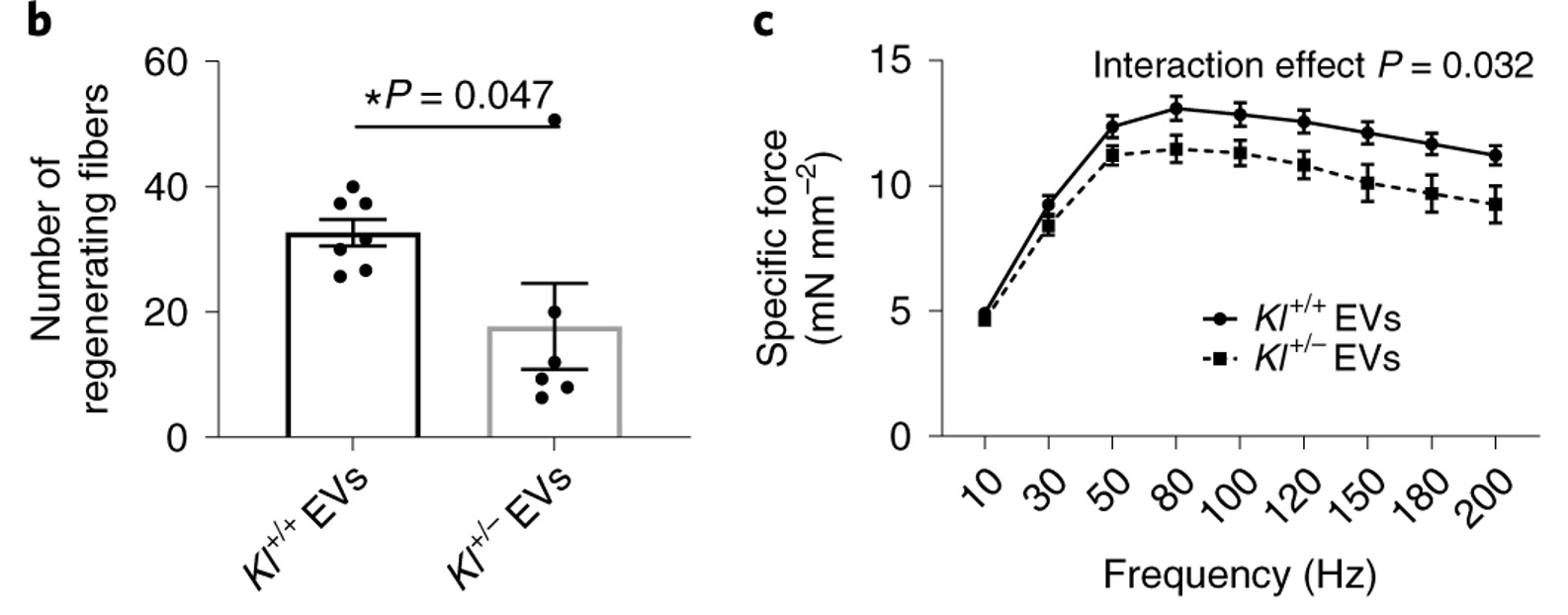
To show that the beneficial effect of young EVs on muscle regeneration is dependent on Klotho transcript levels, Sahu and colleagues EVs isolated from young mice that either had half of the genetic material encoding Klotho deleted or were genetically unaltered were injected into old mice, which contained significantly reduced levels of Klotho mRNA. Compared to those that received EVs from mice with the complete set of genetic material, aged animals receiving EVs from young mice missing half the genetic material encoding Klotho displayed a significant decrease in large myofibers, increased muscle fiber scarring (fibrosis), and impaired force recovery.
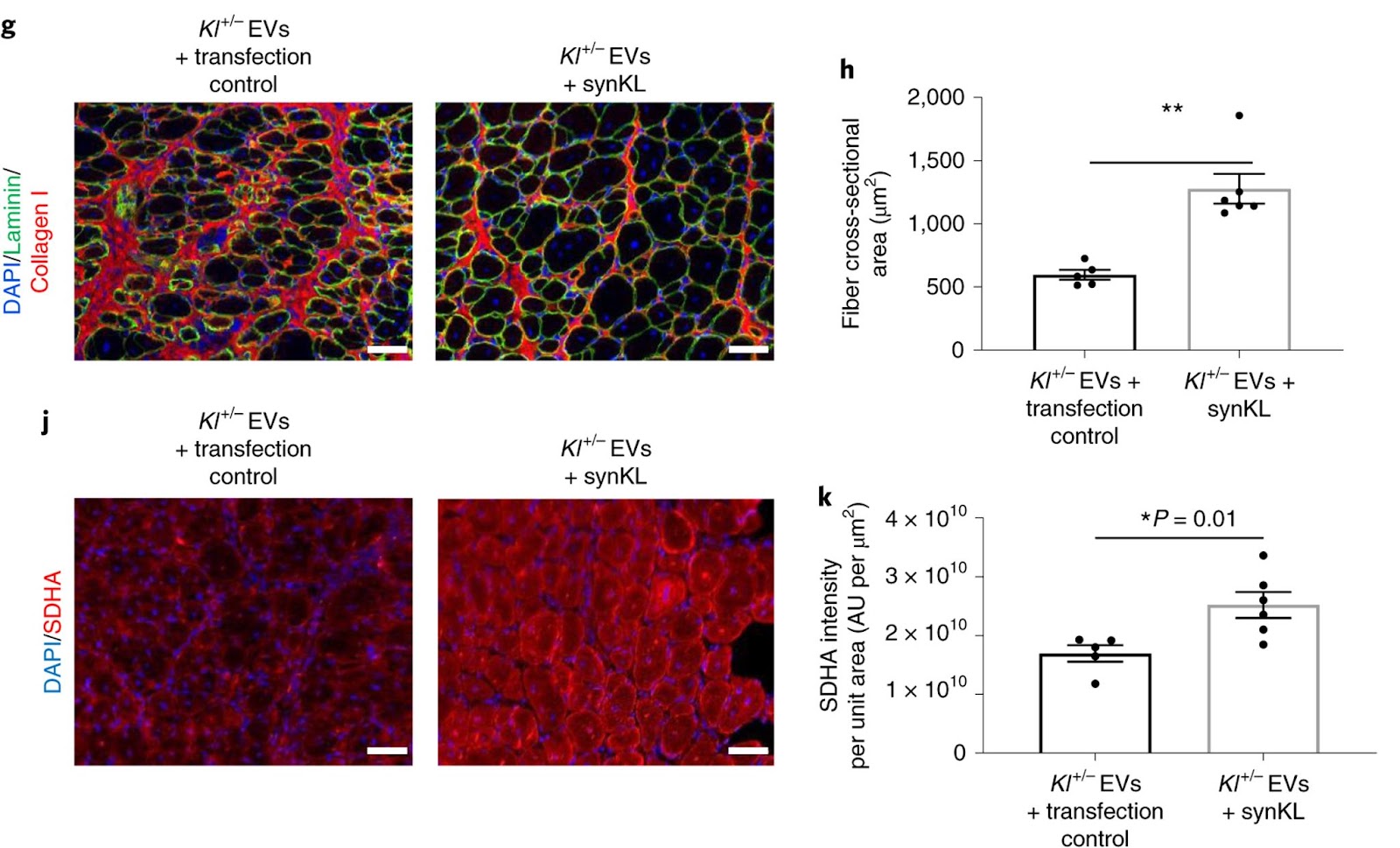
Flipping this experiment on its head, Sahu and colleagues took the mice with half of the genetic material encoding Klotho and engineered them so that the EVs would generate synthetic Klotho mRNA. When the UPMC and University of Pittsburgh researchers injected injured aged mice with these genetically engineered EVs, they observed significantly improved myofiber size and reduced fibrosis when compared to their counterparts missing Klotho genetic material. Taken together, these findings support the conclusion that Klotho transcripts within circulating EVs promote functional skeletal muscle regeneration.
What’s Next for EVs and Muscle Regeneration?
Together, these findings suggest that Klotho transcripts within circulating EVs contribute to the systemic regulation of aged skeletal muscle regeneration. “EVs may be beneficial for boosting regenerative capacity of muscle in older individuals and improving functional recovery after an injury,” said Ambrosio.
She says that he is really excited about engineering EVs with specific cargoes so that we can dictate the responses of target cells. An enhanced mechanistic understanding of the potential of circulating EVs to attenuate, prevent, or even reverse age-related declines in skeletal muscle regeneration may help pave the way toward the development of EV-based therapeutic approaches to the benefit of the growing aging population.

