Preliminary Findings from Clinical Trial Confirm Senolytic Therapy’s Safety for Alzheimer’s
This initial test using dasatinib plus quercetin suggests that senolytic therapy in patients with Alzheimer’s disease is safe and will encourage larger-scale trials addressing key issues.
Highlights:
- This 12-week trial tested intermittent senolytic therapy on 5 participants with early-stage symptomatic Alzheimer’s.
- The study supports the safety and tolerability of senolytic combo dasatinib and quercetin in older adults with Alzheimer’s
- While the study was not meant to test for efficacy, the results suggest that senolytic treatment can affect senescence biomarkers in the cerebrospinal fluid as well as the blood plasma.
A senolytic therapy is being tested in a phase 1 clinical study, which is a first step toward finding new and better ways to treat Alzheimer’s disease. Researchers at the University of Texas Health Science Center in San Antonio have released the results of SToMP-AD, the first clinical trial to test a senolytic therapy (meant to get rid of old cells in the brain) on people with early-stage Alzheimer’s disease. The combination of dasatinib and quercetin did not change blood pressure, brain imaging tests, or cognitive tests. However, the results of this Nature Medicine study on senolytic therapy in Alzheimer’s disease patients will lead to more large-scale trials that focus on important issues.
The search for new Alzheimer’s drugs
New phase 3 Alzheimer’s trials that focused on stopping the buildup of harmful proteins that cause the disease slowed down cognitive decline and the progression of the disease (based on certain neurodegenerative biomarkers). Despite the fact that this is a significant advancement in the field, the clinical effects are still quite minimal, and the risk-benefit ratio will still be considered in terms of the risk of brain edema and the atrophy of the brain due to treatment. So, the search for more therapeutic approaches is still very important.
It is still thought that getting older is the biggest risk factor for Alzheimer’s disease. This has led to a lot of interest in finding ways to stop age-related processes, especially cellular senescence. Getting rid of senescent cells with senolytics, which are drugs that only kill senescent cells, has a lot of clinical potential. Right now, two of the best-studied senolytics are dasatinib, a drug approved by the Food and Drug Administration (FDA) for chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL), and quercetin, a flavonoid found in plants that has anti-inflammatory, antioxidant, and anticancer properties. When dasatinib and quercetin are used together, they have been shown to get rid of only senescent cells in culture, in animal models, and in people.
SToMP-AD Phase 1 Results
Because preclinical research showed strong support and human studies of dasatinib plus quercetin for other diseases showed good safety profiles, researchers conducted the first clinical trial of senolytic therapy for Alzheimer’s disease. The study was supervised by Miranda E. Orr, PhD, who was previously at the University of Texas Health Science Center in San Antonio and now is an Associate Professor with her own lab at the Wake Forest University School of Medicine.
The main goal of the study was to find out how well dasatinib and quercetin worked in the central nervous system (CNS). They also wanted to know about other things that happened, like safety and feasibility, the senolytic compounds’ ability to do their job, and changes in biomarkers in the cerebrospinal fluid (CSF) and plasma that were linked to Alzheimer’s disease, cognition, neuroimaging, and functional status. They enrolled five participants with early-stage symptomatic Alzheimer’s disease in an open-label 12-week intervention of intermittent senolytic therapy and provided the first report of the trial outcomes.
Dasatinib was not present in plasma or CSF before treatment. After the intervention, dasatinib was detected in the plasma of all five participants. In CSF, post-treatment dasatinib levels were slightly above the limit of quantitation in four of five participants. Quercetin, which is found in many fruits and vegetables, was not detectable in three patients and at extremely low levels in two patients at baseline. Following treatment, quercetin was detected in plasma across participants. Within CSF, quercetin was not detected either before or after treatment across participants.
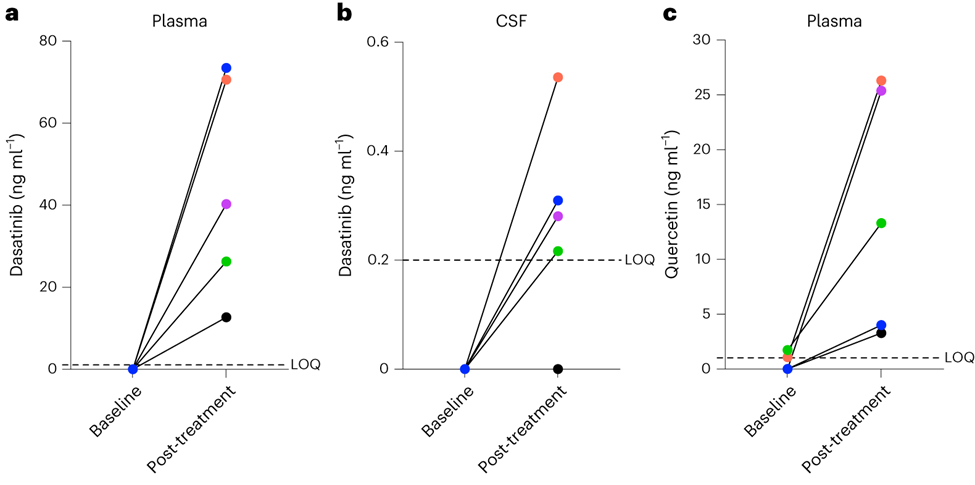
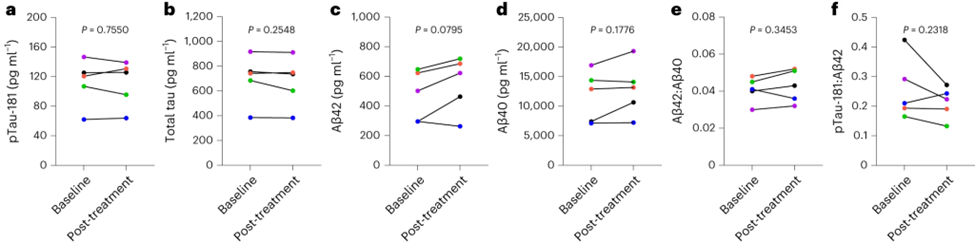
Consistent with the small sample size and brief duration of the study, no effects on cognition or structural MRI measures were observed. Also, there were no statistically significant results for the effects of dasatinib plus quercetin on biomarkers for Alzheimer’s disease and related dementias. The researchers noted that the patients showed an increase in several molecules that are indicative of cells dying and bursting open. But it is unclear how many of these cells were being cleared from the CNS versus from the periphery, especially since there was no detectable quercetin in the CNS.
Considerations for Future Trials
For future studies, it might be important to find out how big of an effect cellular senescence might have at different stages of the disease and how well a balance can be struck between the healing effects of getting rid of inflammatory and other harmful factors and the effects of getting rid of potentially helpful parts of the SASP (like neurotrophins). To be more complete, future research should also look into the interesting questions of whether lowering inflammation related to aging could have short-term benefits and how long any disease-modifying effects might last.
The people who wrote this paper agree that Alzheimer’s trials need to last a lot longer than this one in order to be able to be interpreted at all. They appear to be trying to formulate quercetin in a way that will allow it to reach deeper into the CNS, but they have already begun a placebo-controlled trial in these conditions.
Model: Human, Five participants (mean age = 76 + 5 years; 40% female)
Dosage: Injection with D+Q

