One-Time Injection Enhances Brain Function In Monkeys
Researchers from Yale School of Medicine and UCSF discovered that a single jab of the longevity factor klotho improved the memory of aged non-human primates.
Highlights:
- The non-human primate form of klotho is stable, safe, and increases blood levels of klotho protein in aged rhesus macaques.
- A single, low-dose injection of klotho enhances cognition in aged rhesus macaques in as little as 4 hours and lasts two weeks.
- This work suggests that a systemic low-dose klotho treatment may prove therapeutic in aging humans.
Researchers were able to improve the cognitive function of old monkeys with just a single subcutaneous injection of a specific protein called klotho. These monkeys were the same age as 65-year-old humans, indicating that these results support using klotho in clinical settings to improve cognitive performance in the elderly. Members of the labs of Graham V. Williams and Dena B. Dubal at the Yale School of Medicine and the University of California, San Francisco (UCSF) carried out the research, which was published in Nature Aging.
Klotho Is a Longevity Factor in the Mouse, Monkey, and Man
The protein klotho was discovered in klotho-deficient mice, which developed a syndrome similar to human premature aging. Since then, klotho has been linked to a variety of molecular signaling pathways and diseases. Klotho has anti-aging, healthspan and lifespan-extending, cognitive-enhancing, anti-oxidative, anti-inflammatory, and anti-tumor properties.
Klotho improves cognitive performance through acute peripheral administration, and systemic elevation of Klotho in mice improves synaptic plasticity, cognition, and neural resilience to aging and neurodegenerative diseases like Alzheimer’s and Parkinson’s. However, it is unclear whether treatment with Klotho could improve cognition in elderly non-human primates.
Studies show that people with higher levels of Klotho, whether from a variation in the DNA encoding the klotho protein or other causes, have improved cognitive abilities, reduced neuropathological measures, and a lower risk of developing dementia associated with aging and Alzheimer’s disease. Because of this, there has been a lot of interest in making a drug that boosts or restores klotho and in using klotho levels in the body as a biomarker to help diagnose and treat diseases.
The Yale School of Medicine and UCSF researchers wanted to see if aging rhesus macaques would benefit from klotho’s cognitive enhancement effects like mice do. Similar to humans, rhesus macaques experience cognitive decline as they age, but unlike humans, synaptic changes rather than significant neuronal loss are the cause of this decline.
The main goal of the study, led by Stacy A. Castner of Graham V. Williams’ lab at Yale School of Medicine, was to see if they could improve cognitive performance in rhesus macaques by giving them a dose of klotho that would raise serum levels to those seen in humans throughout their lifespan and be comparable to therapeutically effective increases in mice.
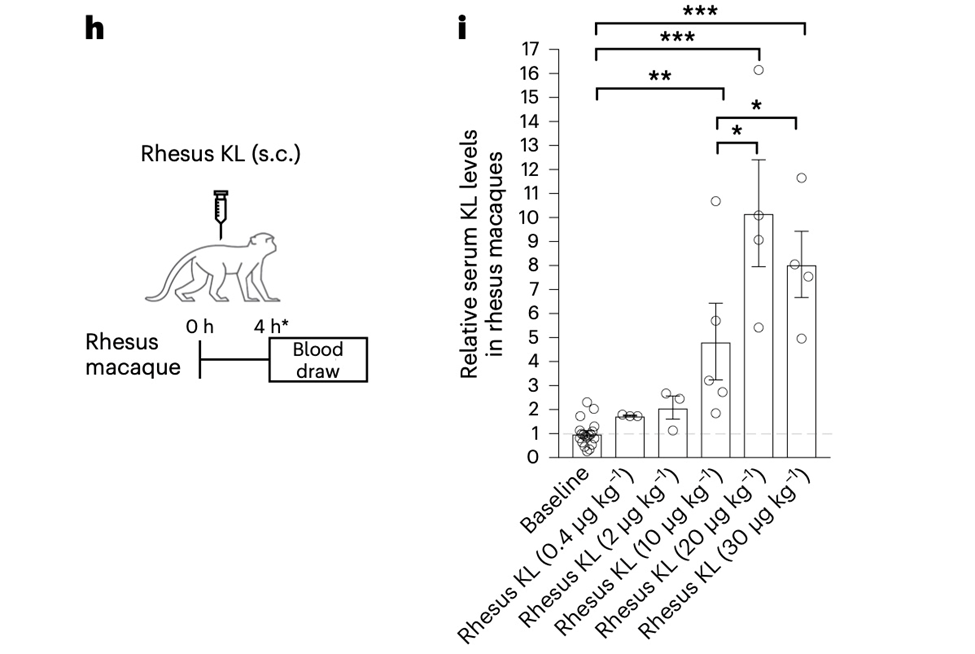
To accomplish this, they administered a single subcutaneous dose of vehicle or rhesus klotho to aged rhesus macaques (average age of 21.78 years, which is the human equivalent of 65 years) and then tested the animals’ cognitive abilities. The primary analysis focused on a dose of 10 g/kg klotho because it produced klotho increases comparable to those found naturally in humans and mice.
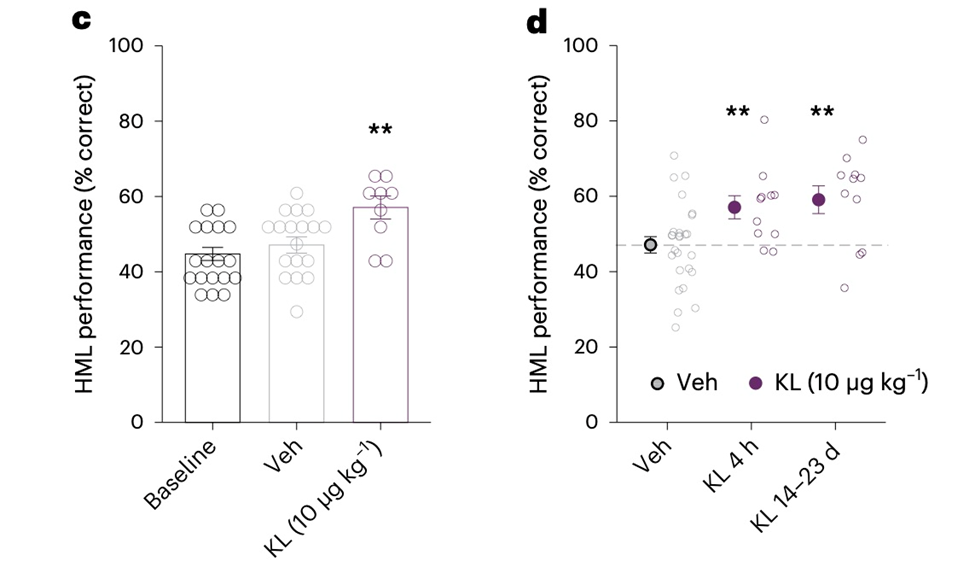
The Yale and UCSF teams used the spatial delayed response (SDR) task to evaluate the cognitive abilities of senior rhesus macaques, specifically the performance of fronto-temporal circuits and regions of the brain like the hippocampus and prefrontal cortex. The SDR test evaluates working and spatial memory under both low (NML) and high (HML) memory loads.
Rhesus macaques treated with a single injection of 10 g/kg klotho showed improved HML performance just 4 hours later, mirroring the rapid improvement in mouse cognition. At 2 weeks, the benefits of klotho-mediated cognition enhancement in HML were still noticeable. This dose of klotho also improved typical NML performance, and the improvement persisted across various tests for the first two weeks following the treatment’s end. There was no sex difference in the klotho-mediated improvement.
Their secondary objective was to investigate whether or not the beneficial effects of klotho on cognition are dose-dependent by administering it to rhesus macaques at higher doses. A higher dose of klotho did not improve monkey brain function, even at 30 micrograms per kilogram. It is important to note that the higher doses tested did not impair cognition, as the 2-5% changes were not statistically increased or decreased. Nonetheless, it is still unknown whether doses even higher than those tested could impair cognition.
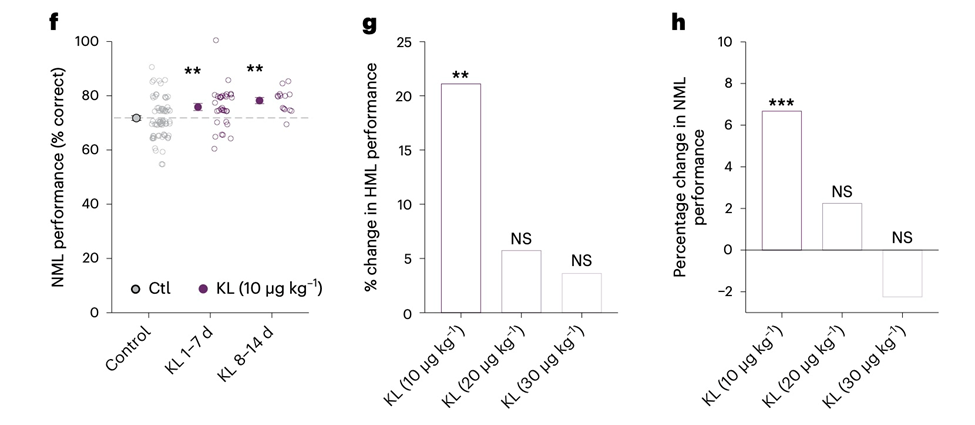
Collectively, these findings suggest that non-human primates, which share a complex genetic, anatomical, and functional brain with humans, also benefit from klotho-mediated cognitive enhancement. These findings also imply that a therapeutic window of cognitive enhancement in humans may require lower, more ‘physiological’ levels of the hormone in the body.
Klotho Clinical Trials
The first concrete evidence of the age-dependent change of soluble klotho levels in healthy subjects was demonstrated in a study published in 2010. In the 13 years since, there have been no human clinical trials involving the use of klotho protein as a therapeutic agent. Over 20 clinical trials involving klotho as a biomarker for aging and disease are currently underway or have been completed.
The FIT-AGING clinical trial, for example, was completed in December 2017 to determine the effect of different exercise modalities on Klotho protein and the physiological consequences of activating the Klotho gene in sedentary humans. The CHANGE clinical trial, which was completed in March 2020, looked at the effect of a low-calorie diet on klotho levels, brain stem cells, and cognition. And a clinical trial is currently underway to determine whether serum levels of klotho can be used as a biomarker of peripheral artery disease progression. However, the findings of any of these studies have yet to be published.
While there is hope for klotho as an aging treatment and biomarker, not all published research on the topic has supported klotho’s relevance in aging-related diseases, particularly kidney diseases. There has been research that shows a significant association between Klotho and kidney function, but there have also been inconsistent findings that suggest Klotho may not be a good surrogate biomarker in chronic kidney disease. This means that much more research is needed to determine the clinical significance of Klotho in chronic kidney disease.
Having baseline values of klotho in healthy and diseased individuals across the lifespan will be essential for developing such treatments. As we know more about klotho, the possibility for a klotho-boosting therapeutic becomes more real.
Model: rhesus macaques (mean age = 21.78 years; putative human age = 65 years)
Dosage: 10 μg/kg, 20 μg/kg, or 30 μg/kg rhesus klotho (KL)

