Northwestern University Study Shows NMN Improves Motor Neurons in ALS Mice
Nicotinamide mononucleotide (NMN) improves the health of motor neurons that become diseased due to ALS, shows a study published in Scientific Reports.
Highlights
- In a mouse model of amyotrophic lateral sclerosis (ALS), defects begin to occur early in the movement controlling centers of the brain, including reduced levels of NAD+.
- Treatment with the NAD+ precursor NMN improves the health of motor neurons from the ALS mouse model.
- Proper modulation of metabolomic imbalance via NAD+ precursors could offer therapeutic strategies to improve the health of motor neurons in ALS patients.
Besides a few extraordinary cases, such as that of the late English Theoretical Physicist Stephen Hawking, the neurodegenerative disease amyotrophic lateral sclerosis (ALS) has a life expectancy of just a few years and still has no cure. ALS, commonly referred to as Lou Gherig’s disease, is driven by problems with a protein called TDP-43 in upper motor neurons — the nerves that carry signals away from our brain to control our muscles. Inside these diseased upper motor neurons are dysfunctional mitochondria, fundamental cell structures for metabolism and energy generation.
A study from Northwestern University published March 11, 2022, now shows that a reduction in NAD+ — an essential metabolite in our bodies — underlies the mitochondrial dysfunction in ALS. Published in Scientific Reports, the researchers demonstrate that treatment with the NAD+ precursor NMN improves mitochondria’s integrity and the health of cultured upper motor neurons from ALS mice. “Our results show that metabolomic defects occur early in ALS motor cortex and establishing NAD+ balance could offer therapeutic benefit to UMNs with TDP-43 [disease],” wrote the study authors Mukesh Gautam, Ph.D., and colleagues.
Mitochondrial Dysfunction Drives Metabolic Changes in ALS
The primary causes of ALS and the disrupted processes in sick neurons are the subject of intense research. Becoming quite clear is that the upper motor neurons from ALS patients and mouse models of TDP-43 disease — initially defined by the accumulation of TDP-43 proteins in degenerating neurons — exhibit significant mitochondrial abnormalities. Since mitochondrial defects have been found in TDP-43 driven ALS e, one of the critical questions that remain unanswered is: “What are the metabolomic changes that occur concerning TDP-43 disease in the context of ALS?”
To answer this, Gautam and colleagues first analyzed the metabolite profile of both healthy and diseased motor cortex — the brain region that relays voluntary movement — to investigate whether metabolomic changes occur in a mouse model for TDP-43 disease. They selected an early symptomatic stage of the disease to examine whether metabolomic perturbations begin to appear early in the motor cortex with TDP-43 disease. Their findings revealed the TDP-43 diseased motor cortex had reduced energy (ATP) production and changes in metabolites – such as phosphoenolpyruvate, glutathione, 2-hydroxyglutarate, and NAD+.
NMN Improves the Health of Neurons Affected by ALS
It is well known that metabolic dysregulation leads to depletion of NAD+, affecting energy balance. NAD+ levels can be restored by compounds such as NR (nicotinamide riboside), NAM (nicotinamide), NA (niacin), and NMN (nicotinamide mononucleotide). These precursors are converted to NAD+, which can be readily used for energy generation. Restoration of NAD+ levels has been beneficial and neuroprotective in Alzheimer’s disease and intracerebral hemorrhage. Moreover, treating a different mouse model of ALS called hSOD1G93A with NR slowed down the degeneration of upper motor neurons and reduced neuroinflammation.
With this in mind, Gautam and colleagues tested if increasing NAD+ levels could help restore balance and improve the health of upper motor neurons affected by ALS driven by TDP-43 disease. Since NMN (nicotinamide mononucleotide) has been reported to increase the levels of NAD+ in neurons, the Northwestern University researchers used NMN to increase NAD+. To do so, they took upper motor neurons from the brains of TDP-43 disease mice and grew them in lab dishes with NMN. They found that treatment with NMN improved neuronal health and the mitochondrial integrity of TDP-43 disease upper motor neurons.
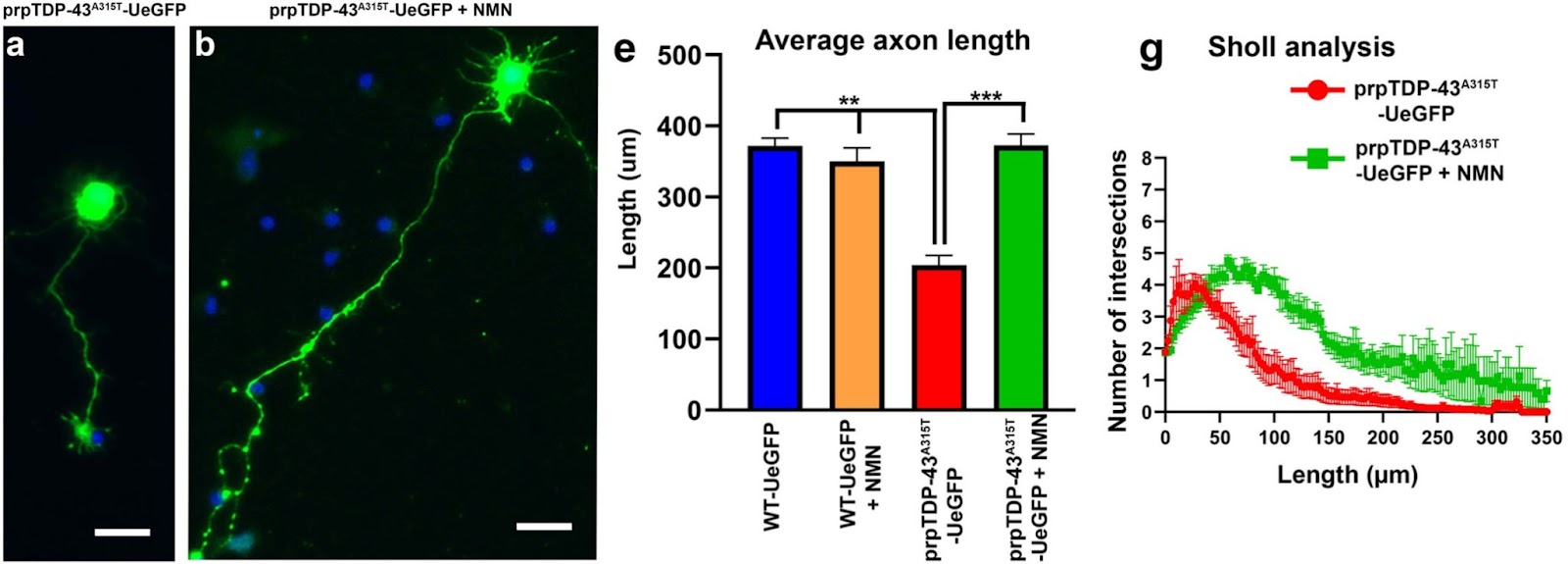
(Gautam et al., 2022 | Scientific Reports) Nicotinamide mononucleotide (NMN) improves the health of upper motor neurons that become diseased due to TDP-43 disease. Representative images of cultured upper motor neurons from TDP-43 disease mice (a) untreated or (b) treated with 1 µM NMN. From these images, the (e) average small projection (neurite) length and (g) branching (arborization) of the small projections (Sholl analysis) were calculated. Increases in projection length and branching (as determined by the number of intersections) indicate improved upper neuron health.
What’s Next for NMN Treatment of ALS?
Even though long-term exposure to NMN may be harmful to axonal stability and neuron health due to its interaction with an enzyme called SARM1, maintaining NAD+ balance appears to be a vital contributor to upper motor neuron health, particularly in those with TDP-43 disease. As a result, these findings highlight the critical necessity of understanding the metabolomic issues that arise early in the sick motor cortex and promote the development of innovative treatment techniques to restore the brain’s disturbed NAD+ levels.

