NMNH Raises NAD+ Substantially More than NMN
An NMN molecule with an added hydrogen atom, NMNH, boosts blood NAD+ about twice as much as NMN and significantly elevates NAD+ in tissues where NMN does not—muscle, the heart, and the brain—in mice.
Highlights
- Reduced nicotinamide mononucleotide (NMNH) substantially increases blood nicotinamide adenine dinucleotide (NAD+) in a sustained manner compared to nicotinamide mononucleotide (NMN) in mice.
- Tissues where NMN has not been shown to increase NAD+, such as muscle, the heart, and the brain, show about 20% to 30% more NAD+ with NMNH.
- Despite these promising preclinical study findings, there are no ongoing or planned human trials to test NMNH’s efficacy in alleviating age-related diseases.
NAD+ plays a critical role in numerous cellular reactions and processes such as DNA repair and energy generation, and its cellular levels decline with age in animal models and humans. Relatedly, Shin-Ichiro Imai, an aging expert from Washington University in St. Louis, has referred to this age-related systemic decline in NAD+ as a key driver of aging.
NAD+ precursors like NMN and nicotinamide riboside (NR) have been used to increase systemic NAD+ to counteract age-related physical deterioration associated with falling NAD+ levels. As such, using NMN and NR to replenish NAD+ has been shown to alleviate age-related cardiovascular, metabolic, and neurological conditions in mice; however, human trials have given mixed results.
Interestingly, a more potent form of NMN, NMNH—an NMN molecule with an additional hydrogen atom—works better to boost NAD+ than other NAD+ precursors, at least in mice. Accordingly, if NMNH has this effect in humans, the possibility looms that this molecule may replicate some of the aging intervention properties seen in animals for people.
As presented in a Modern Healthspan YouTube video, NMNH nearly doubles the amount of NAD+ in the blood of mice compared to NMN. Not only that but while blood NAD+ levels taper off after about 20 hours with NMN treatment, NMNH provides a sustained NAD+ elevation over a 24-hour time course.
Even more substantial, in tissues where NMN does not increase NAD+, like the muscles, heart, and brain, NMNH significantly boosts NAD+. This could be important, because in Shin-Ichiro Imai’s NAD World 3.0 model of aging, falling NAD+ in a brain region he labeled as the control center of aging—the hypothalamus—plays a significant role in aging. Thus, boosting NAD+ in the brain with NMNH may increase hypothalamic NAD+, and if Imai’s theory holds, this could slow age-related physiological deterioration.
Even with animal studies showing NMNH increases NAD+ better than other NAD+ precursors, there are still no recruiting or ongoing human trials to test whether it works to counteract age-related diseases. Moreover, NMNH has been declared as generally recognized as safe (GRAS) in the US by a biotech company called Effepharm. This legally recognized GRAS label declares NMNH safe based on Effepharm’s evaluations without a formal Food and Drug Administration (FDA) review, which the FDA can still challenge.
Hence, due to Effepharm’s self-affirmed GRAS that purportedly assures NMNH’s safety, why no research on NMNH has been done since 2021 remains unclear. Furthermore, the lack of human trials on NMNH remains unclear, also, but may be due to such studies being cost-prohibitive.
High NMN Doses Used for Mouse Studies May Not Work in Humans
Difficulties translating the aging intervention effects of NAD+ precursors in mice may stem from dosage issues as animal studies have used high doses. For instance, mouse studies have often used 500 mg/kg per day of NMN, which translates to approximately 3 grams for an average human. This high dosage for humans raises concerns as NMN can be converted to the molecule nicotinamide, which is toxic to the liver at high doses.
Furthermore, many NMN products only give approximately 500 mg to 1 gram of NMN daily, and due to NMN’s conversion to nicotinamide, the higher doses shown effective in mice may not be ideal for humans. A crucial question arises from this dosage conundrum: can we identify a molecule that boosts NAD+ more effectively than NMN without triggering high nicotinamide levels? The answer may be the molecule NMNH, warranting a closer look at research related to this molecule.
Along these lines, two studies have been published regarding NMNH. In the first, NMNH injections increased NAD+ faster than NMN. Moreover, NMNH nearly doubled blood NAD+ compared to NMN injections, and while NAD+ level elevations from NMN tapered off after about 20 hours, NMNH treatment triggered a sustained increase in NAD+ for at least 24 hours. The dosages used in these mice were 250 mg/kg injections or about 1.5 grams for adults weighing about 165 pounds. These results suggest that NMNH more effectively increases blood NAD+ compared to NMN.
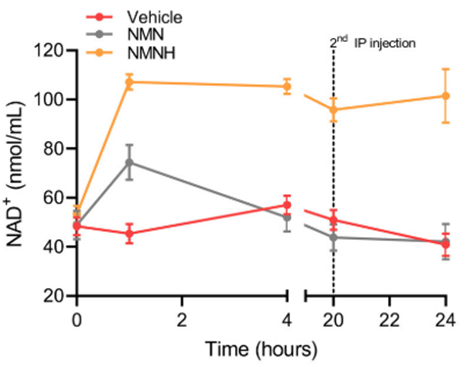
Furthermore, in the same study, NMNH significantly boosted NAD+ in muscle, the heart, and the brain, tissues where NMN did not. This finding begs the question of whether NMN’s lack of efficacy in counteracting age-related diseases in humans may arise, in part, from not increasing tissue NAD+ in muscle, the heart, and the brain. Along those lines, if these organs have lower tissue levels of NAD+ with age, this may facilitate an array of age-related metabolic, cardiovascular, and neurological conditions.
The second study testing NMNH examined the molecule’s effects on human liver cells and mice. In liver cells, NMNH increased NAD+ levels five-fold compared to non-treated cells and almost five-fold compared to NMN-treated cells. Similar results were found for NAD+ levels in the liver of mice—NMNH increased NAD+ four-fold compared to non-treated mice and doubled NAD+ compared to NMN-treated mice. These findings add evidence that NMNH increases NAD+ more effectively than NMN.
Data from the same study, from experiments in human liver cells, also suggest that NMNH inhibits the tricarboxylic acid cycle and glycolysis, two cellular processes involved in cell energy generation. This effect would be adverse to the body’s energy supply if it applies to humans who take NMNH. Interestingly, when Modern Healthspan asked NAD+ pathway expert Dr. Joseph Baur from the University of Pennsylvania about this finding, he said that the slowing of these energy-producing cellular processes may be short-lived.
Dr. Baur hypothesized that levels of NAD+ with an additional hydrogen atom, NADH, increase in cells after NMNH treatment. Elevated NADH could then directly inhibit the tricarboxylic acid cycle after entering the cell’s powerhouse—mitochondria—-following NMNH treatment. Since NADH is a byproduct of the tricarboxylic acid cycle in generating cell energy, increasing NADH after NMNH treatment would temporarily slow the tricarboxylic acid reactions.
Dr. Baur also said that since this data is from cell culture experiments, it remains unclear whether this inhibition would happen in live animals like mice or humans. Additionally, if this effect does happen in living animals after NMNH treatment, researchers do not know if this effect on cell energy generation would last long enough to inhibit metabolism.
No Planned or Ongoing Human Trials Testing NMNH
To find whether NMNH increases NAD+ in more tissues than NMN and whether such an effect would counteract age-related conditions in humans will require clinical studies. However, a quick online search of ongoing clinical trials with NMNH in the US yielded no results. Thus, it can be assumed that no human research is being conducted on NMNH’s effects on aging in humans, and none are recruiting. The reasons behind this lack of NMNH research in humans remain unknown.
Furthermore, NMNH has received a self-affirmed GRAS in the US from the Effepharm biotech company, so it does not seem likely that no human studies are underway due to safety concerns. For background, there are two ways to get GRAS status for a substance: one is to request it from the FDA by providing documentation of its safety, and the other is to self-affirm it with research experts at a company or institution. Along these lines, the FDA can challenge Effepharm’s self-affirmed GRAS for NMNH at any time.
Waiting for More Data on NMNH
Not much new data has been released regarding NMNH since the release of two studies in 2021. With NMNH’s ability to more effectively increase blood NAD+ than NMN in mice and with its ability to boost NAD+ in muscle, the heart, and the brain, finding whether these effects translate to humans will be paramount. Furthermore, if Dr. Joseph Baur’s hypothesis is correct, perhaps people who take NMNH will not need to worry about metabolism inhibition from this molecule.
Finally, it may be that increasing NAD+ across tissues does work to alleviate age-related conditions in humans and that we have only used NAD+ precursors which do not effectively boost NAD+. If that is the case, NMNH may serve as a new way to replicate NAD+ precursors’ effects against age-related diseases as seen in animal models. Only human trials can test whether NMNH can recapitulate NMN’s effects in animals for people, and since none are planned or ongoing, it remains unclear how long we will have to wait for human data.


