Metro Biotech Publishes MIB-626 NMN Human Data
A study published by a research team from Metro International Biotech and Harvard Medical School shows that a pharmaceutical-grade preparation of NMN known as MIB-626 increases blood levels of NAD+ in one week in middle-aged and older adults.
Highlights
- MIB-626 is a pharmaceutical-grade tablet of NMN from Metro International Biotech.
- This study evaluated the ability of varying oral MIB-626 doses to raise intracellular NAD+ levels.
- Two 500 mg MIB-626 tablets taken once or twice daily for two weeks doubled NAD+ blood levels in the participants middle-aged and older adults.
While several studies in mice and human cells on NAD+ enhancement have shown encouraging results, human studies examining the effectiveness of these compounds are scarce. That may be in part due to the fact that there is not a consensus for the appropriate and safe dosages of NAD+ precursors like nicotinamide mononucleotide (NMN). This leaves little reason to roll out clinical studies examining the use of NAD+ boosters to treat medical disorders until such assessments are completed.
Pencina and colleagues from Harvard Medical School’s Brigham and Women’s Hospital showed that 1000 mg of pharmaceutical grade NMN (MIB-626) taken in a tablet once daily or twice daily for two weeks was safe and associated with substantial dose-related increases in blood NAD+ levels. This study — funded by a research grant from Metro International Biotech, a company co-founded by Harvard aging and longevity researcher Dr. David Sinclair and that made headlines for their work with testing their NAD+ boosting product with the US Special Forces — should facilitate the design of efficacy trials for NMN in disease conditions.
The NAD+ Precursor Status Quo
NAD+ levels decrease with age, and this loss has been connected to age-related illnesses. Raising NAD+ levels extends longevity and improves health in mice, as shown by preventing obesity, diabetes, and fatty liver disease, protecting the heart from damage, and stimulating liver regeneration. Furthermore, increasing intracellular NAD+ improves mitochondrial energetics and aerobic capacity.
As a result of these animal studies, increasing NAD+ levels with NAD+ precursors that are derivatives of vitamin B3 — such as niacinamide, NMN, and nicotinamide riboside (NR) — are being investigated as potential therapies to prevent and possibly cure age-related disorders. It is because of these animal studies that sales of over-the-counter (OTC) NAD+ boosters have skyrocketed,which is unsurprising condsidering the implications of such a potentilaly powerfull drug.
Despite encouraging animal and human cell study (preclinical) results, clinical studies using NAD+ precursors have shown mixed outcomes, owing to the variability of study demographics, dosing regimens, limited sample sizes, and short intervention periods. In several early investigations, the dosages employed were relatively modest.
NMN Enhances NAD+ Levels in Humans
The Harvard Medical School researchers tested the safety and tolerability of MIB-626 — a microcrystalline formulation of NMN that comes in 500 mg tablets — in overweight or obese, medically stable, middle-aged and older adults. In addition, Pencina and colleagues looked into the ability of varying doses of MIB-626 to raise intracellular NAD+ and compounds related to NAD+ metabolism.
They found that 1,000 mg taken once or twice daily for 14 days was safe and well-tolerated. There were no serious adverse events, and mild adverse events were similar among groups. Only one subject assigned to a 1000 mg daily dose showed some alterations in the blood levels of liver enzymes, but this was also the case for one of the subjects receiving placebo. The researchers did not find a major effect of sex, body mass index (BMI), and age, although participants’ age and BMI range was narrow and the sample size was small.
For both the once daily and twice daily regimens, 1,000 mg of MIB-626 was associated with substantial dose-related augmentation of blood NAD+ levels. After 14 days, 1,000 mg of NMN taken once daily doubled NAD+ levels and, when taken twice daily, tripled NAD+ levels. This study provides important information about the time course of blood NAD+ level changes and the metabolism of NMN after administration.
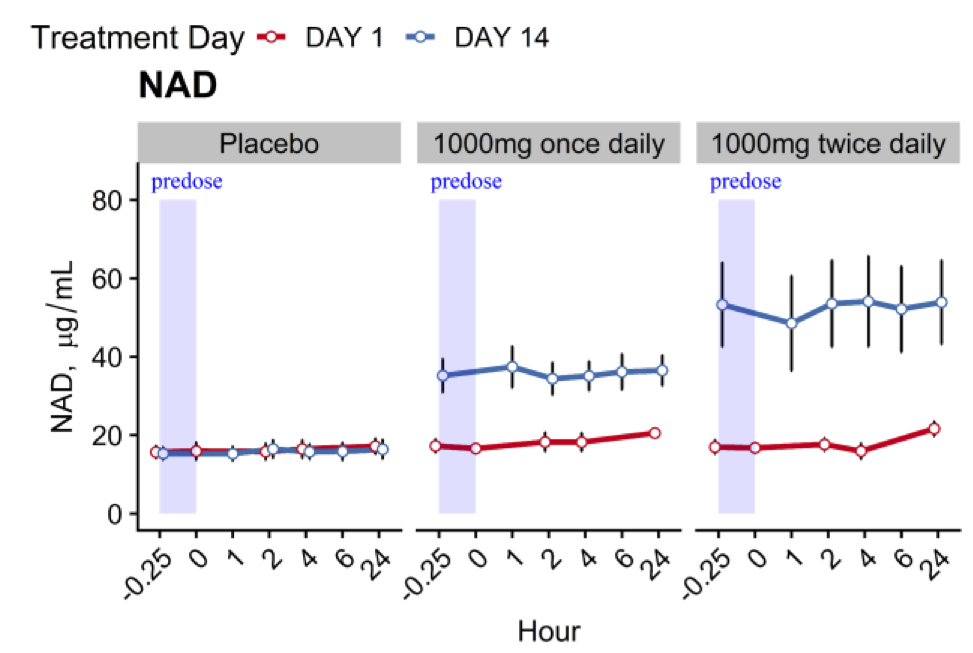
(Pencina et al., 2022 | The Journals of Gerontology) Pharmaceutical-grade tablets of NMN increase NAD+ levels in middle-aged and older adults. The placebo capsules had no effect on NAD+ levels over the course of 14 days (left graph). NMN doses of 1,000 mg taken once daily doubles NAD+ levels (middle graph). When taken twice daily, NMN triples NAD+ levels (right graph).
These increases in blood NAD+ concentrations were linked to substantial increases in the circulating levels of key metabolites, notably nicotinamide and two related compounds 2-PY and 1-methyl nicotinamide. Nicotinamide is the direct byproduct of NAD+ metabolism. Normally, if nicotinamide intake is slightly more than the body needs, excess nicotinamide will be detoxified rapidly and eliminated mainly via the 1-methylnicotinamide to 2-PY pathway, which involves liver and skin functions. The metabolite 2-PY was the most abundant circulating and urinary metabolite, suggesting high levels of NAD+ metabolism.
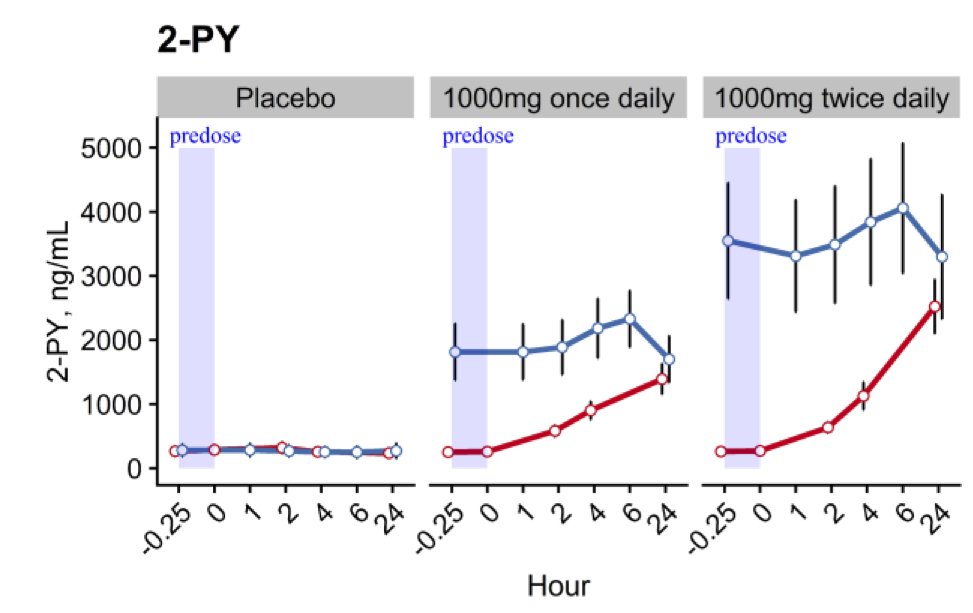
(Pencina et al., 2022 | The Journals of Gerontology) Pharmaceutical-grade tablets of NMN increase levels of the NAD+ metabolite 2-PY in middle-aged and older adults. The placebo capsules had no effect on the levels of the NAD+ metabolite 2-PY over 14 days (left graph). NMN doses of 1,000 mg taken once daily (middle graph) and twice daily (right graph) for 14 days (blue line) generated increases over 10-fold in the levels of the NAD+ metabolite 2-PY. Effects were detected after just one day of NMN treatment (red line).
When Pencina and colleagues looked at blood sugar, insulin, fat, and uric acid levels, amongst other compounds, they didn’t detect any differences. That being said, the trial was neither large enough nor long enough to detect differences in glucose metabolism and insulin sensitivity.
These data are consistent with other reports that also have not found significant effects of NMN or NR on glucose, insulin, fats, and uric acid during short-term treatment. A randomized trial of 250 mg of NMN once daily for 10-weeks did not find significant changes in glucose and insulin levels but reported improvements in other insulin-related processes.
The Next Steps for NMN in Clinical Studies
It is important to note that this early phase trial was neither designed nor powered to evaluate efficacy outcomes. However, the study suggests that subsequent efficacy trials should use NMN regimens of 1000 mg or higher. Doses less than 1000 mg of NMN were not shown to consistently raise NAD+ levels in healthy volunteers, and several recently completed clinical trials used NMN doses much lower than this. For example, a completed clinical trial in India tested the effect of taking 600 mg NMN for 60 days on biological aging, BMI, and walking endurance (NCT04823260), although the results are not yet published.
It will be interesting to see how other pharmaceutical-grade NMN products can increase NAD+ levels in humans. If the dosage results with MIB-626 can be replicated with other NMN formulations, we will likely see clinical trials using NMN in the aging and longevity space begin to take off from all sorts of companies working on NAD+ boosters.
That being said, Metro International Biotech is going ahead and recruiting for at least two phase 2 trials on MIB-626 treatment and has another one listed as not yet recruiting focusing on Alzheimer’s disease (NCT05040321). One actively recruiting clinical study (NCT05038488) will examine MIB-626’s tolerability and effectiveness in adults with COVID-19 infection and stage 1 acute kidney injury in preventing worsening of kidney function and in attenuating the inflammatory response to the infection. A second trial (NCT04817111) will test the safety and tolerability of short-term therapy with a nicotinamide adenine dinucleotide (NAD+) precursor (MIB-626) in adults with Friedreich’s Ataxia (FA). If the efficacy, safety, and bioavailability are demonstrated in the two phase 2 trials, we will likely see larger and longer duration randomized clinical trials that could support a drug registration for NMN.

