NMN Improves Stem Cell Therapy for Injured Aged Mouse Hearts
Combining NMN supplementation with hormone-treated bone-derived stem cell transplantation synergistically increased cardioprotection
Highlights
· Mesenchymal stem cells (MSCs) preconditioned with the hormone ghrelin improved the protection of aged mouse hearts subjected to injury.
· Injecting NMN with the ghrelin-treated MSCs synergistically improved heart repair after injury.
The transplantation of cells found in the bone marrow called mesenchymal stem cells (MSCs), which are important for repairing muscle tissues, is a promising therapy. In particular, MSCs have some protective effects on the heart against injury from ischemia-reperfusion (IR) — when blood stops and then starts flowing into the heart. Optimizing the conditions for MSCs transplantation is key to improving this cell-based therapy, especially in elderly subjects where MSCs seem to be less effective.
In a study published by Sun and Zhang in the European Journal of Pharmacology, MSCs treated with ghrelin, an endogenous growth hormone, transplanted to aged mice subjected to IR injury improved the protective effect of MSCs similar to that seen in young mice. What’s more, when the research duo combined injection of nicotinamide mononucleotide (NMN) with the ghrelin-treated MSCs, the cardioprotective effect increased synergistically.
“NMN supplementation and concomitant administration of preconditioned MSCs is a promising strategy that will protect the aged heart well against IR injury,” proposed Sun and Zhang.
Comorbidities complicate heart injuries
Protecting the heart from IR injury during comorbidities, including aging, is clinically important. In older age, the severity of IR injury appears to be greater than in youth, and studies have shown that the power of the protective effects of therapeutic interventions is severely reduced.
MSC transplantation is a promising therapeutic approach that leads to major reductions in blood supply obstruction (infarct) size in young animals with IR injury. However, after isolating MSCs from original tissues, it’s hard to maintain their capacity for renewal and differentiation, because it’s not the same as the environment inside a living person. As a result, they lose a portion of their therapeutic effects due to high rates of cell death and low rates of survival.
NMN supplementation as a complement to cell-based therapies
NMN, a precursor of NAD+, can protect the hearts of healthy young rats against IR damage and reduce the infarct size. This is thought to occur in part by NMN’s ability to stimulate the production of mitochondria — our cells’ energy generators — and even enhancing the cell’s recycling processes (autophagy). Since the function and number of mitochondria are severely reduced in aging, mitochondria-targeted compounds like NMN may improve the aged heart’s response to other interventions.
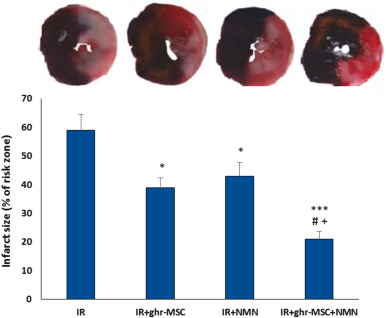
NMN and hormone-treated MSCs synergistically reduced infarct size in aged hearts
Because myocardial IR injury in the presence of comorbidities has multifactorial nature, there is increasing emphasis on the use of combination therapy in this disease. Combination therapy can overcome the reduced response to interventions in the elderly and reinforce cellular defense mechanisms more sustainably and strongly.
For this purpose, Sun and Zhang added NMN with ghrelin-treated MSCs and evaluated their effect on infarct size. Ghrelin, a growth hormone we all make, might be a factor that contributes to the cardioprotective effects of MSCs. Ghrelin may also lead to the greater durability of MSCs effects in the infarcted heart by reducing inflammatory responses and promoting mitochondrial function. So, the research duo treated aged rats (20 to 22 months old) with IR injury with ghrelin- or unconditioned-MSCs at early reperfusion. NMN (500 mg/kg, i.p) was also administered at early reperfusion and repeated 12 hours later.
Although NMN alone had a significant effect on reducing infarct size similar to the ghrelin-treated MSC group, their combined application could significantly and more potently reduce infarct size compared to the IR group. The impact of combination therapy on the reduction of infarct size was significantly greater than those of individual treatments. The combination of ghrelin-treated MSCs plus NMN supplementation was able to synergistically enhance each other’s effect and provide strong protection of the aged heart against IR injury.
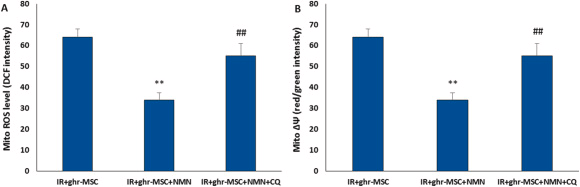
After adding NMN to the MSCs treatment regimen, mitochondrial function was re-evaluated by examining the changes in reactive oxygen species production and membrane potential. The results showed that the effect of combination therapy on decreasing mitochondrial reactive oxygen species and increasing mitochondrial membrane potential was greater than the effect of ghrelin-treated MSCs alone.
“Ghrelin may serve as a promising candidate to improve the cardioprotective efficacy of MSC-based therapy via autophagy/mitochondrial pathway and that NMN serves as a good booster in combination therapy in aged hearts,” concluded the authors.
Finding other survival mechanisms involved in this effect, as well as identifying the contribution of each of them to combination therapy-induced cardioprotection will lead us to find more effective and central therapeutic targets.

