NMN Relieves Despair from Social Stress, New Study Shows
In the face of social defeat, NMN may counteract social avoidance, lack of pleasure, and despair.
Highlights:
- 100 mg/kg of NMN fed to mice for 3 weeks reduces depressive-like behavior induced by social defeat stress.
- NMN increases NAD+ levels and cellular energy in a region of the brain associated with depression.
A new study suggests that social stress leads to lower NAD+ levels in a region of the brain that regulates emotions and stress called the frontal cortex. The decrease in NAD+ appears to lower the production of cellular energy by mitochondria, leading to depressive-like symptoms. However, restoring NAD+ levels with NMN mitigates this depressive-like behavior.
NMN Prevents Depressive-Like Behavior from Social Stress
To model depression in mice, researchers employed the social defeat stress paradigm. This paradigm involves placing a test mouse — the intruder — into the home cage of a larger, more aggressive breed of mouse — the aggressor. Upon entering the cage, the intruder is immediately attacked and dominated by the aggressor.

As such, the researchers subjected mice to the social defeat stress paradigm 10 days in a row — chronic social defeat stress (CSDS). As a result, the mice exhibited depressive-like behavior, including social avoidance, lack of pleasure, and despair. However, in mice fed 100 mg/kg of NMN for 3 weeks before CSDS, these depressive-like behaviors were largely prevented.
Social Avoidance
To measure social avoidance, mice were placed into an unfamiliar area with a small, empty cage at one end. The mice’s movements were then tracked for 2.5 minutes. Next, an aggressor mouse was placed into the cage and the mice’s movements were tracked again. The less time spent near the cage in the aggressor’s presence was considered avoidant social behavior.
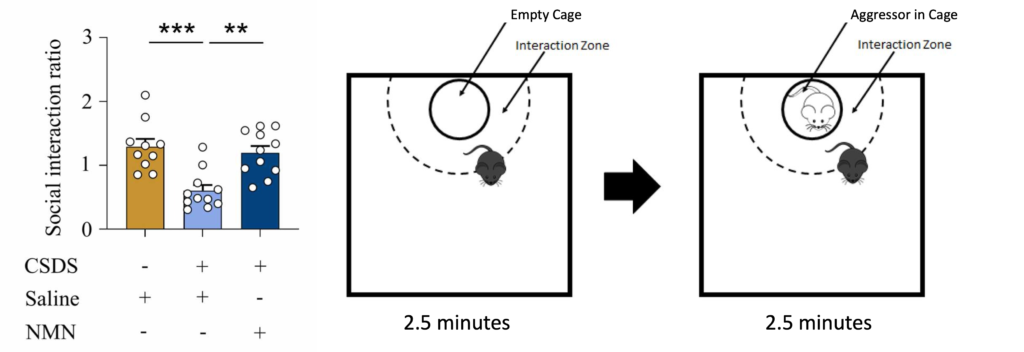
(Left graph: Deng et al., 2024, Right diagram: Wendelmuth et al., 2020) NMN Prevents Social Avoidance. Left: Compared to unstressed mice (gold), mice who experienced chronic social defeat stress (CSDS, light blue) exhibit less social interaction. However, NMN (dark blue) prevents this decrease. Right: Social interaction was measured by the amount of time spent in the interaction zone in the presence vs absence of the aggressor.
Lack of Pleasure
To assess a lack of pleasure (anhedonia), the researchers measured the mice’s preference for sucrose, also known as table sugar. Mice naturally prefer sweet food and will choose water containing sugar over water without sugar. However, mice exposed to varying forms of stress do not choose sugar water over water. The reason for this could be that stressed mice no longer gain pleasure from the sweet taste of sugar water. Accordingly, the researchers found that CSDS reduced the mice’s preference for sucrose. However, treatment with oral NMN prevented this loss of preference.
Despair
To evaluate despair —the loss of hope, the researchers applied the tail suspension test. For this test, mice were suspended by their tail approximately 1.6 feet above the floor. In this type of situation, mice will naturally attempt to escape and move about sporadically, but in depression models, they stop struggling and become immobile. Immobility can be interpreted as giving up on escaping. Thus, CSDS increased the duration of time mice were immobile. However, NMN treatment prevented this increase.
Can Early Life NMN Deter Depression and Dementia?
Depression in early- (age 18-44) or middle-age (age 45-59) is associated with an increased risk for dementia later in life. Therefore, limiting depression throughout one’s lifespan could potentially reduce the risk of dementia in old age. While a previous study showed that NMN reduces depressive-like behavior in mice, whether NMN can mitigate depression in humans requires further study. Still, a previous study showed that NAD+ therapy alleviated clinical symptoms of depression in 93% of 205 patients, suggesting NMN could do the same. Additionally, the hypothetical basis for NMN and NAD+ counteracting depressive symptoms seems sound.
The New Biology of Depression
For the past 50 years, it has been thought that depression is caused by a lack of serotonin and norepinephrine. However, this hypothesis may be a gross oversimplification of the neurobiology of depression. The mitochondrial hypothesis posits that dysfunctional mitochondria cause depression. Mitochondria produce cellular energy called ATP and the brain demands more energy than any other organ.
In the brains of depressive patients, mitochondria are dysfunctional and lack an important protein called Complex I in regions like the frontal cortex. This leads to the generation of reactive oxygen species (ROS), which causes cellular damage. Furthermore, impaired mitochondria produce less ATP and can initiate cell death. This may explain the degeneration of the frontal cortex observed in depressed patients.
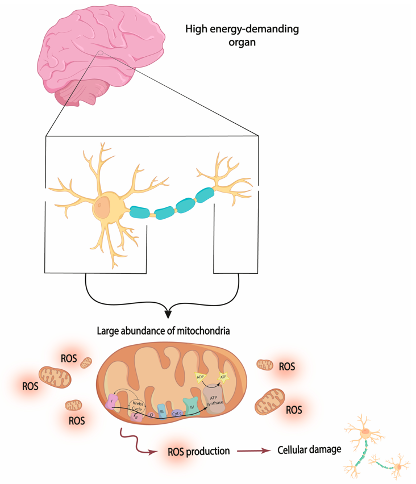
(Larrea et al., 2024) Mitochondrial Hypothesis of Aging. Dysfunctional mitochondria in the brain of depressed patients produce reactive oxygen species (ROS), which damage cells and disrupt normal brain function.
NAD+ facilitates the production of ATP by directly interacting with Complex I in mitochondria. Thus, by increasing NAD+ levels, it is possible that mitochondria can function more efficiently and produce more ATP. Furthermore, most ROS are produced by Complex I, so NAD+ may also reduce ROS levels. In this way, boosting NAD+ with NMN could mitigate mitochondrial dysfunction while counteracting damage and death to neurons in regions of the brain like the frontal cortex. This may result in improved emotional and stress regulation and reduction of depressive thoughts.

