Chinese Researchers Show NMN Rejuvenates Brain Cells In Aged Mice
Researchers from Zhejiang University School of Medicine show that the NAD+ precursor NMN enhances the ability of brain cells to generate myelin — the protective insulation coating nerve projections — in naturally and prematurely aged mice.
Highlights
- Levels of an enzyme called SIRT2 and NAD+ are substantially reduced in aged oligodendrocyte precursor cells (OPCs) — myelin generating cells, preventing neurons from being myelinated efficiently.
- Supplementing with NMN restores SIRT2 and rejuvenates aged OPCs, promotes their maturation, and enhances myelination in the aged central nervous system.
- The elevation of NAD+ levels via NMN injection restores remyelination in prematurely aged mice subjected to a demyelinating injury.
Much like an electrical wire is encased with a plastic coating, most of our nerve projections, known as axons, are covered in a protective, insulating sheath called myelin. But, with age, this fat-based wrapping begins to decay — a process known as demyelination. That’s because aging disrupts the ability of oligodendrocyte progenitor cells (OPCs) to mature, which is essential for the remyelination of exposed nerve cells.
A Nature Communications study published March 9, 2022, by Xiao-Ru Ma and colleagues from Zhejiang University School of Medicine in China reports that the protein SIRT2 — an enzyme that depends on the vital molecule NAD+ to function — is critical for preventing demyelination. However, during aging, levels of SIRT2 and NAD+ decline, preventing the maintenance of myelin promoted by OPC maturation. SIRT2 also gets stuck in the cytoplasm, preventing it from carrying out its role in differentiating OPCs into mature oligodendrocytes. Elevating the NAD+ levels of aged mice via injection of the precursor NMN (10 mg/kg bodyweight) restores SIRT2 levels, including its re-entry into the nucleus of OPCs, promotes oligodendrocyte maturation, delays myelin aging, and enhances remyelination in the central nervous system.
(Ma et a., 2022 | Nature Communications) Elevating NAD+ rejuvenates aged oligodendrocyte precursor cells to promote remyelination. NAD+ targets oligodendrocyte precursor cells (OPCs), restores SIRT2 nuclear localization in aged OPCs, delays myelin aging, and enhances myelin repair following demyelination in the aged central nervous system.
Demyelination and Brain Disorders
Demyelination is a significant component of nervous system inflammatory illnesses like multiple sclerosis and an early marker of neurodegenerative diseases. What’s worse is that this means demyelination can lead to the permanent degeneration of axons. Throughout life, oligodendrocytes mature from OPCs and remyelinate axons, but the effectiveness of remyelination decreases with age, owing to a decrease in OPC maturation capability. Multiple sclerosis causes degeneration due to this impairment in maturation, typically lasting several decades and worsens with age. When multiple sclerosis enters a progressive phase, it is almost untreatable. Finding novel molecular targets to revitalize aging OPCs offers a lot of potential for this unmet medical need.
OPCs are found throughout the central nervous system and are critical in myelination and remyelination. Sirtuins, enzymes dependent on NAD+, are thought to play a role in the aging of OPCs. However, which of the seven sirtuin family members are expressed in OPCs remains a mystery. Sirtuin 2 (SIRT2) is the sole member primarily found in the cytoplasm, and its function in the central nervous system is unknown. Although mature oligodendrocytes produce SIRT2, it is unclear if OPCs in the central nervous system do the same or what function SIRT2 plays in the remyelination of the elderly central nervous system.
NMN Promotes Remyelination In Aged and Injured Mice
Compared to any of the other six sirtuin family members, Ma and colleagues found that the gene activity of SIRT2 was dominant in OPCs grown in a dish. However, gene activity does not tell the whole story, as the SIRT2 protein (product of gene activity) was found in the nucleus of OPCs during myelin formation but was absent in OPCs from the adult central nervous system. Indeed, SIRT2 localization shifts when going from OPCs to mature oligodendrocytes during development. In mice, the researchers found that demyelination induces the re-entry of SIRT2 in the nucleus of OPCs in the adult central nervous system, resembling earlier stages of development. SIRT2 re-entry was impeded in old mice, which corresponded to poor remyelination.
Also in mice, SIRT2 was in the nucleus while myelination occurred during development after birth, and this was recapitulated in the adult central nervous system in response to demyelination. This led the China-based research team to study the role of SIRT2 in the OPCs of mice by selectively deleting SIRT2 in OPCs. The deletion of SIRT2 lowered the frequency of remyelinated axons, decreased the thickness of newly created myelin, and reduced the frequency of normal-looking freshly generated myelin.
Following that, Ma and colleagues showed that increasing NAD+ with NMN supplementation via injection (10 mg/kg bodyweight) once daily slowed myelin aging and improved myelin repair in the central nervous system of old mice. The Chinese research team discovered that NAD+ was one of the most depleted metabolites in old OPCs using a premature aging mouse model. Upon NMN injection in a dosage equal to the clinical study in which the long-term clinical safety of NMN was established, the researchers discovered that NAD+ repletion restored SIRT2 nuclear entry and delayed myelin aging in the aged OPCs.
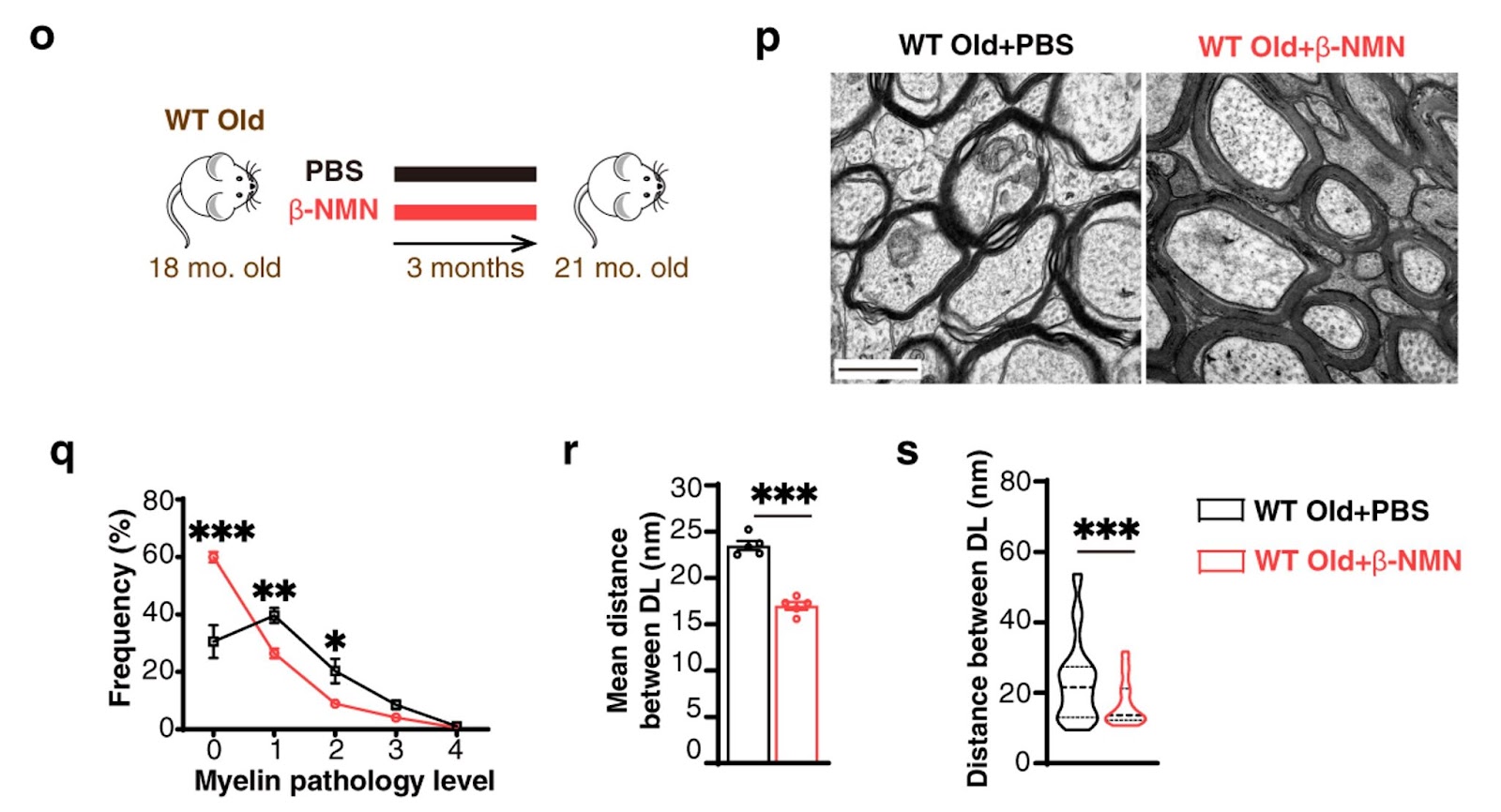
(Ma et a., 2022 | Nature Communications) NMN delays myelin aging in aged mice. (O) β-NMN was once daily injected into naturally aged old mice for three months. (P) Microscopic images of myelin in the part of the brain that connects both hemispheres (corpus callosum) of naturally aged mice to distinguish unambiguously the myelinated, demyelinated (no myelin), or remyelinated (newly formed myelin, thinner) axons within the lesion. Quantification of (Q) myelin pathology level, with “0” being no pathology, and (R, S) the distance between the dense line (DL) – a reliable way to evaluate myelin thickness and compaction.
Furthermore, the Chinese research team demonstrated that NAD+ boosting via NMN supplementation improves remyelination efficiency in the aged central nervous system is a preventive and therapeutic one. In a premature aging model, NMN injection (10 mg/kg bodyweight) induced demyelination and made newly formed myelin, which was thicker, more compact, and, therefore, higher quality. Together, these results show that NMN supplementation significantly enhances remyelination efficiency in premature aging mice.
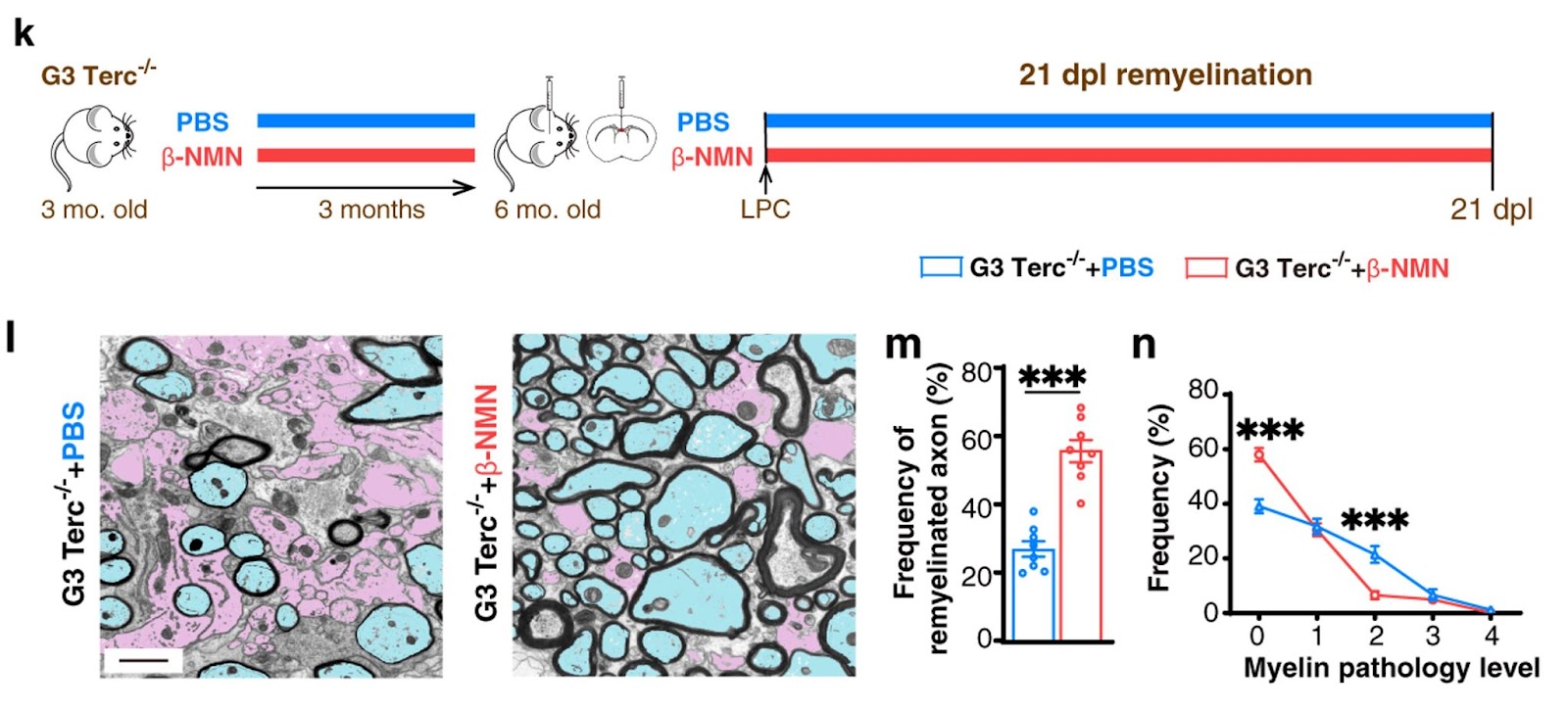
(Ma et a., 2022 | Nature Communications) NMN enhances remyelination in aged mice. (K) Ma and colleagues tested whether NAD+ repletion could enhance remyelination in premature aging mice (G3 Terc−/−). (L) Axons are colored in pink for demyelinated ones and blue for remyelinated ones. These results showed that at 21 days post-lesion (dpl), NAD+ repletion by β-NMN injection in prematurely aged mice (M) doubled the frequency of remyelinated axons as well as (N) increased the frequency of normal myelin (grade 0) and generally decreased the myelin pathology level in comparison with untreated prematurely aged mice (grade 1-4).
Can NMN Treat Demyelinating Disorders like Multiple Sclerosis?
Other research has shown that SIRT2 protein levels decreased within the brain lesion of both the multiple sclerosis model and patients. In light of that, the results in this study by Ma and colleagues highlight the potential for this work to go towards clinical translational studies, bringing hope to potential treatments for the progressive phase of multiple sclerosis, a so-far unmet therapeutic challenge. Recent studies have shown that in young mice, supplementing with NAD+ decreases the disease score in a multiple sclerosis model. Additionally, supplying niacin, an indirect NAD+ precursor, beneficially modulates the immune system, suggesting that NAD+ has versatile targets. This work expounds on NAD+’s role in myelin health and the ability of NAD+ precursors like NMN in brain rejuvenation.

