NMN Reduces Inflammation and Defends Against Viruses: Japanese Study
NMN reduces the activation of inflammatory and NAD+-consuming proteins in a human cell model of viral infection.
Highlights:
- NMN mitigates viral-mediated inflammation by preventing the elevation of mRNA for pro-inflammatory IL-6.
- NMN aids in defending cells against viruses by altering levels of mRNA for NAD+-dependent PARP9.
Older folks are more susceptible to viral infection and exhibit higher mortality rates from viruses than younger folks. Such a conundrum may be exacerbated by deficiencies in how our cells battle viruses, which requires energy. Since nicotinamide adenine dinucleotide (NAD+) promotes energy production but declines with age, scientists from SBX Biosciences in Japan investigated the effect of boosting NAD+ on cellular antiviral defenses.
In Scientific Reports, Sano and colleagues show that NMN prevents the activation of a pro-inflammatory protein called IL-6 in artificially-infected human cells. Additionally, they show that NMM reduces the activation of a protein called PARP9, suggesting a win on the so-called “NAD+ battlefield,” which involves PARP9 having sufficient levels of NAD+ to overcome viral proteins. These findings suggest that NMN mitigates viral-mediated inflammation and aids in the protection against viruses.
NMN Prevents Viral-Mediated Inflammation
To model viral infection, Sano and colleagues exposed human lung cells to a synthetic chemical called polyinosinic-polycytidylic acid (PPA), which mimics viral infection. Subsequently, the researchers treated PPA-infected human cells with a low (0.1 mM), medium (1.0 mM), or high (10.0 mM) dose of NMN, which is known to replenish cellular NAD+ levels.
Generally speaking, genetic information flows in one direction, from DNA, to messenger RNA (mRNA), to protein. Often, this mRNA is measured to estimate the production of its corresponding protein. It follows that, since we already know the function of most proteins, we can predict how a cell will function based on changes in mRNA levels.
Sano and colleagues found that the mRNA levels of interleukin-6 (IL-6) — a pro-inflammatory protein — were elevated in infected human lung cells, suggesting an elevation in inflammation. Upon treatment with a high-dose of NMN, however, IL-6 mRNA went down to normal levels. These findings suggest that NMN mitigates viral-mediated inflammation.
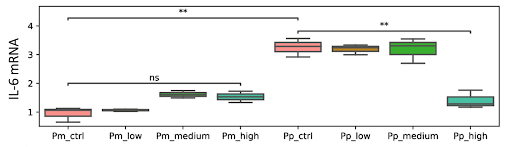
(Sano et al., 2023 | Scientific Reports) NMN Mitigates Viral-Mediated Inflammation. Pro-inflammatory IL-6 mRNA levels are higher in infected human lung cells (Pp_ctrl) when compared to non-infected human lung cells (Pm_ctrl). However, a high dose of NMN (Pp_high) normalizes IL-6 mRNA levels, suggesting the mitigation of inflammation.
The “NAD+ battlefield” involves a battle between human proteins called PARPs and proteins produced by viruses. The NAD+ battlefield is called so because NAD+ is broken down by PARPs, which cleave ADP-ribose molecules from NAD+ molecules. PARPs then attach ADP-ribose molecules to proteins. Viral proteins do the exact opposite and remove ADP-ribose molecules from proteins. Since adding an ADP-ribose to specific proteins leads to destroying viruses, it is critical that PARPs win the battle.
Sano and colleagues found that the mRNA levels of PARP9, a specific form of PARP, were elevated in infected cells. However, a high-dose of NMN reduced PARP9 mRNA levels, suggesting that high PARP levels were no longer necessary. These findings point to NMN aiding in winning on the NAD+ battlefield by boosting NAD+ levels.
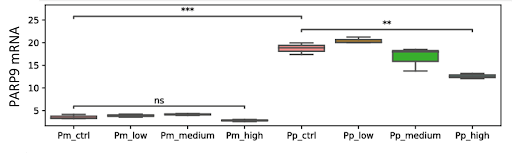
(Sano et al., 2023 | Scientific Reports) NMN Aids in the Cellular Defense Against Viruses. PARP9 mRNA levels are higher in infected human lung cells (Pp_ctrl) when compared to non-infected human lung cells (Pm_ctrl). However, a high dose of NMN (Pp_high) reduces PARP9 mRNA levels, suggesting NMN aids in defending cells from viruses.
NMN: An Anti-Aging Anti-Inflammatory
One key property of NMN highlighted by Sano and colleagues is its ability to suppress inflammation. Chronic inflammation causes damage to organs and tissues and underlies many age-related diseases. Thus, by mitigating inflammation, NMN could also counter one of the primary underlying features of multiple conditions.
While protein levels were not measured by Sano and colleagues, NMN has been shown to reduce pro-inflammatory proteins secreted by immune cells during hyper-allergic reactions in mice. NMN has also been shown to reduce pro-inflammatory factors in the gut to treat inflammatory bowel disease and reduce obesity-associated inflammation in fat tissue. Pro-inflammatory proteins, including IL-6 have also been shown to decrease in response to NMN upon lung injury. More recently, NMN was shown to reduce brain inflammation in a mouse model for sepsis — an overactive immune response that leads to body-wide inflammation. Thus, NMN mitigates inflammation in a wide range of age-related conditions, supporting its role as an anti-inflammatory anti-aging compound.

