NMN Reduces Colitis in Mice
Administration of NMN protects against damaging inflammation in mice modeling colitis.
Highlights
- Deficient NAD+ synthesis in specific immune cells (macrophages) promotes mouse susceptibility to colitis.
- Macrophage cleaning-up activity that engulfs dead cells and repairs tissue is dependent on NAD+ synthesis.
- NMN administration mitigates colitis severity and improves mouse survival.
Inflammation is at the root of the vital immune response to invaders. First, inflammation activates, recruiting an army of immune cells to destroy harmful bacteria or viruses (pathogens). However, inflammation must eventually deactivate because persistent and excessive inflammation can lead to diseases, such as asthma and inflammatory bowel diseases (IBD).
Researchers from Ajou University School of Medicine in Korea have helped clarify the mechanisms underlying inflammation and colitis, finding that levels of NAD+ – a compound critical for cellular energy – are linked to colitis severity in mice. In a study published in Redox Biology, mice lacking NAMPT in immune cells called macrophages, the enzyme that produces an NAD+ precursor called NMN, showed increased colitis severity in response to inflammation as well as decreased survival. These effects, both disease severity and survival, could be rescued by injecting the mice with NMN (500 mg/kg) three times over the course of a week. These findings suggest that NAMPT-dependent NAD+ biosynthetic pathway activation may treat inflammatory diseases like IBD.
Finding the inflammatory balance
Our guts are metabolic metropolises filled with sprawling populations of microbes and cells. One such cell, the macrophage, plays a crucial role in fine-tuning the intestinal mucosal immune system. These intestinal macrophages function to clear pathogens, bacterial wall components, and dying cells.
However, failure to mount a robust protective response against pathogens during the resolution phase of inflammation may lead to persistent and excessive inflammation, as often observed in IBD. Additionally, accumulating evidence suggests that enforcing inflammatory resolution in macrophages might represent a novel therapeutic approach for controlling intestinal inflammation and restoring tissue function.
NAD+ synthesis regulates intestinal inflammation
Nicotinamide phosphoribosyl-transferase (NAMPT) plays an important role in the cyclic biosynthetic pathway of nicotinamide adenine dinucleotide (NAD+), essential for the maintenance of cellular energy. NAMPT catalyzes NMN s, which is a direct precursor to NAD+, and is biologically indispensable and implicated in various inflammatory diseases, including rheumatoid arthritis, diabetes, and sepsis. However, the role of NAMPT in inflammatory macrophages has not been fully elucidated, particularly in the context of IBD.
To investigate the role of NAMPT in IBD, Hong and colleagues induced colitis in mice with macrophages lacking a functional NAMPT gene. This genetic alteration caused mice to suffer from prolonged, severe inflammation, although the initial inflammatory response was preserved regardless of the lack of NAMPT activity. These mice with NAMPT deficient macrophages also exhibited major reductions in body weight and lower survival rates.
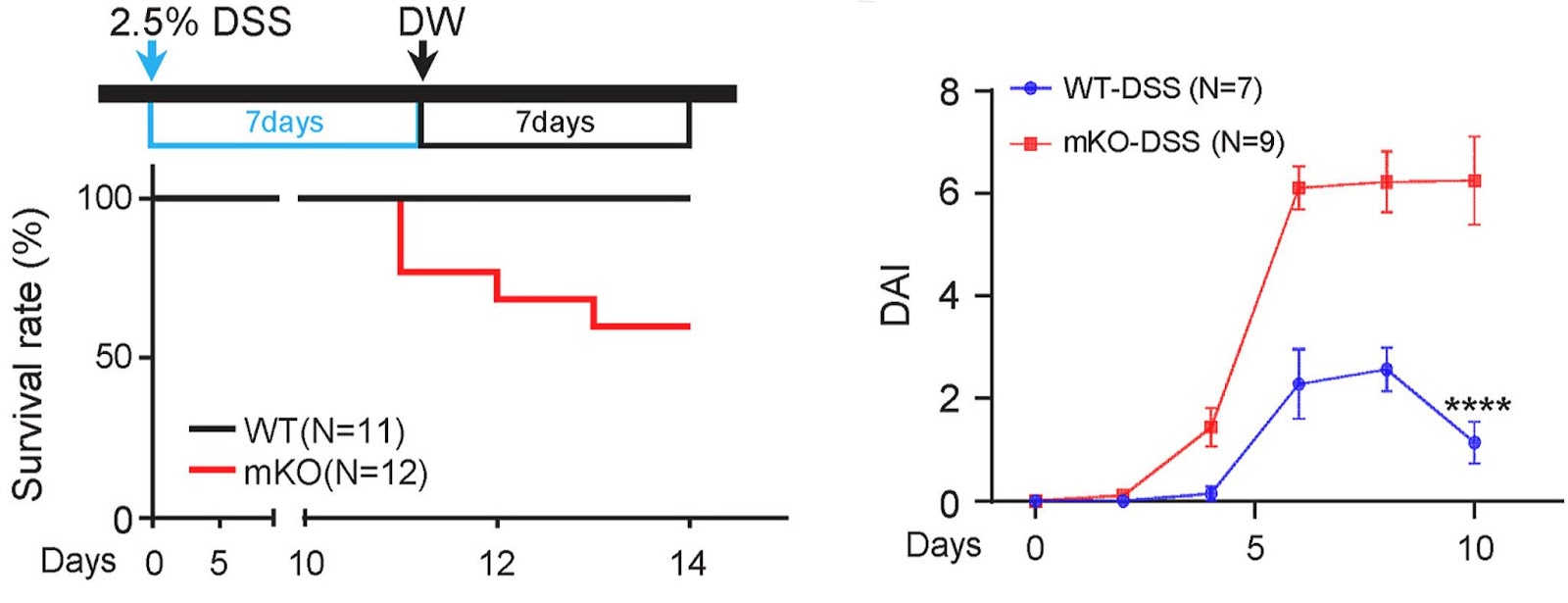
While NAMPT deletion did not affect the recruitment of macrophages or the production of inflammatory cytokines, NAMPT deletion impaired a “clean-up” process (phagocytosis) of macrophages, which is important in the resolution of inflammation and restoration of tissue balance. The reduced phagocytosis impeded clearing tissue debris and dead cells derived from insufficient NAD+ levels. Collectively, these results suggest that NAMPT deletion specifically in macrophages leads to prolonged, severe colitis in a mouse model.
NMN improves survival of mice with colitis
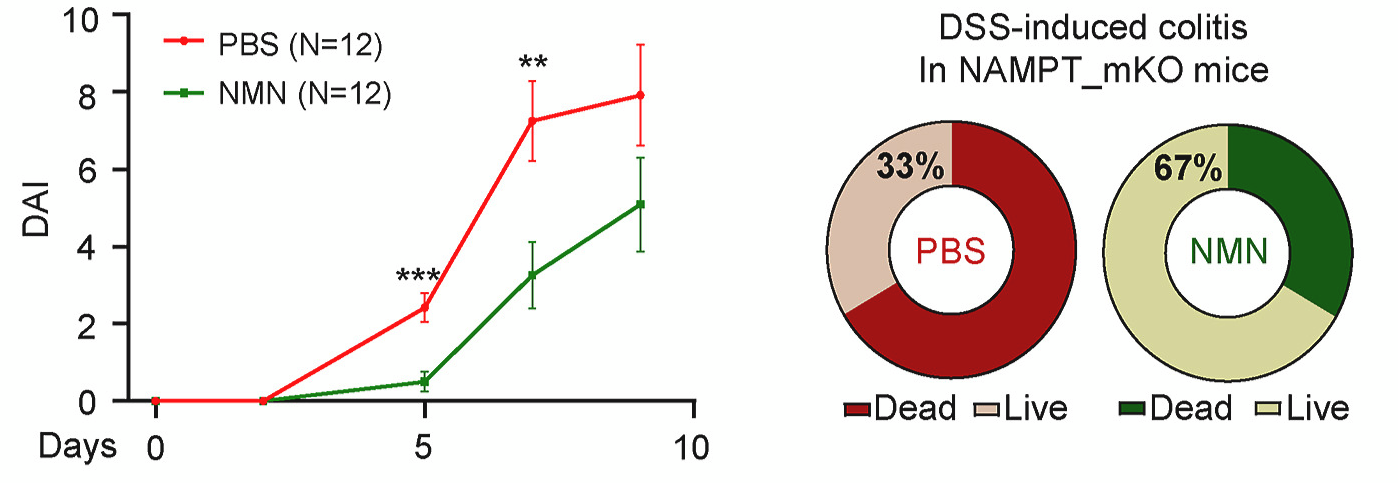
In these same mice, Hong and colleagues injected 500 mg/kg of NMN or saline three times per week. NMN treatment alleviated bodyweight reduction and colitis severity, resulting in a better survival rate. In detail, saline injection resulted in 33% survival (4/12 mice), whereas NMN administration resulted in 67% survival (8/12 mice). Consistently, NMN treatment increased the number of cells in a replicative state while decreasing cell death markers and reducing macrophage infiltration. These results show that NMN administration protects mice from colitis by enhancing the resolution of inflammation.
Next, Hong and colleagues examined whether any toxic side effects accompanied by NMN injection may present in mice. No gross and microscopic abnormalities were observed in the colon, liver, or spleen, and the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were not increased upon NMN treatment, indicating that NMN injection could attenuate colitis without obvious liver toxicity.
“Collectively, our findings suggest that activation of the NAMPT-dependent NAD+ biosynthetic pathway, via NMN administration, is a potential therapeutic strategy for managing inflammatory diseases,” concluded Hong and colleagues.
Can NMN treat IBD?
Despite this new therapeutic proposal, the clinical translational relevance of this study seems to be limited. That’s because the condition related to ‘macrophage-specific NAMPT depletion’ is not expected to be encountered easily in a clinical setting.
Nevertheless, Hong and colleagues emphasize that NAD+ is critical in the cleaning up activity of macrophages and in the subsequent resolution of inflammation. In cells, including macrophages, NAD+ is made mainly from nicotinamide (a form of vitamin B3, also known as niacin), with various dietary sources like fish, meat, and mushroom. Without proper supplementation, people can suffer from its deficiency, a condition known as pellagra.
Meanwhile, patients with IBD commonly have trouble eating or taking anything orally because they frequently go through abdominal pain, diarrhea, or decreased appetite. Malabsorption is another common problem in IBD patients. So, these challenges often result in nutritional imbalance or deficiency. The reported prevalence of malnutrition in IBD patients ranges between 20% and 85%. Furthermore, malnutrition is one of the most critical factors associated with a poor clinical outcome in IBD patients. Thus, this collective information implies that NAD+ deficiency may occur in immune cells – which is equivalent to NAMPT depletion in macrophages in our study – of chronic IBD patients with malnutrition.
In addition, aging is accompanied by a gradual decline in tissue and cellular NAD+ levels in multiple organisms, including humans. This decline in NAD+ levels is tied to numerous aging-associated diseases, such as cognitive decline, cancer, and metabolic disease. Remarkably, it has been reported that NAMPT levels also decrease in many tissues during organismal aging.
Thus, it seems that a decrease in NAD+ is ascribed to the reduced NAMPT levels in aged cells, at least in part. Taken together, supplementation of NMN (or nicotinamide) seems to be an effective treatment strategy that helps to reduce the severity and the duration of inflammation in patients with IBD, in particular those who are old or suffer from malnutrition.

