NMN Protects the Mouse Heart from Ischemia and Reperfusion Injury
Ischemia and reperfusion injury can lead to life-threatening conditions. Scientists found that NMN may be a new intervention for it.
Ischemia and reperfusion (I/R) — when blood stops flowing into the heart (ischemia) and then is followed by a rush of blood flooding into the heart (reperfusion) — is a life-threatening event. The heart damage induced by I/R is an important cause of heart failure in patients.
In a study on PLOS One, a group of researchers led by Junichi Sadoshima from Rutgers New Jersey Medical School discovered that a chemical compound, nicotinamide mononucleotide (NMN), can protect the heart from ischemia and reperfusion injury (I/R injury) in mice.
Heart Facts About I/R
Each day, an average person takes about 20,000 breaths a day without thinking much about it. Every inhalation pulls oxygen into our lungs, where it enters our blood vessels and travels to organs and cells. While arteries are like highways for blood flow, capillaries are tiny alleyways, with some only wide enough for one blood cell to pass at a time. When any of these roads get blocked, body parts can’t get enough blood and oxygen, resulting in ischemia.
Ischemia can happen about anywhere in the body and sometimes leads to life-threatening events such as heart attacks and strokes. About 805,000 Americans have a heart attack every year, according to the Centers of Disease Control and Prevention, meaning that someone has a heart attack every 40 seconds. Conventionally, doctors treat the patient by removing the blockage or creating a bypass to restore blood flow with open-heart surgery.
Restoring the blood flow in ischemia sounds like an easy solution, but it actually can cause more harm than good, leading to a problem called reperfusion injury. Instead of restoring the tissue’s normal function, reintroducing the circulation results in inflammation and oxidative damage through unstable oxygen molecules.
Past studies have shown that during periods of cardiac ischemia, the level of nicotinamide adenine dinucleotide (NAD+), a key molecule in energy production, drops. Scientists also knew that nicotinamide phosphoribosyltransferase (Nampt), an enzyme that helps the production of NMN, raises NAD+ level and protects the heart against I/R injury.
NMN Protects the Heart from I/R Injury
Sadoshima and colleagues found that Nampt plays a role in heart protection by mediating the effects of ischemic preconditioning, which is one of the most potent mechanisms against I/R injury. The mechanism exposes the heart to short episodes of ischemia that allows the organ to build up resistance to the loss of blood supply and oxygen, working up the ability to endure extended periods of ischemia.
Sadoshima and his team discovered that NMN compensates for the adverse effect of I/R injury by mimicking the effects of Nampt.
Both mice with or without ischemia showed an increase in NAD+ level in the heart after NMN injection. The elevation of NAD+ level started as early as 30 minutes after the injection and persisted over an hour, reversing the effect of ischemia on NAD+ content.
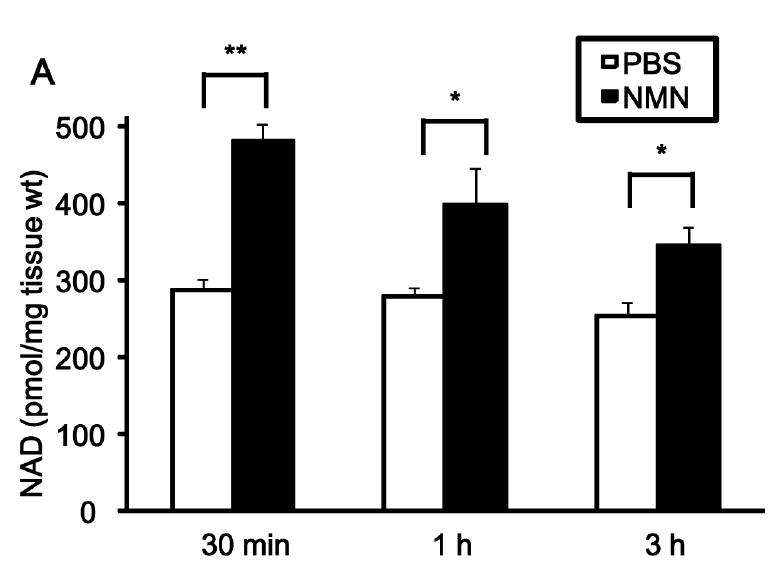
NMN also reduced the myocardial infarct area, a small localized area of dead tissue resulting from the inadequate blood supply to the heart. The compound protected the heart when it was applied once 30 minutes before ischemia or four times just before and during reperfusion, by reducing the infarct size by 44% and 29%, respectively. The research team says that their finding further “confirms the protective effect of NMN against I/R injury.”
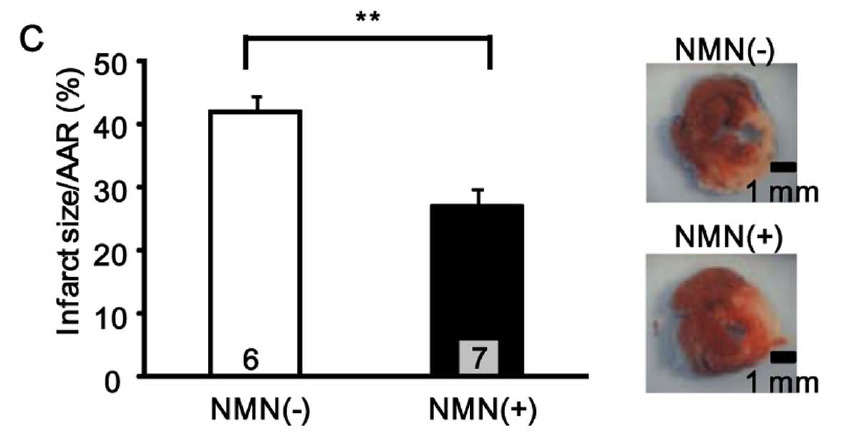
“Although NMN protects the heart from I/R injury, its instability in the systemic circulation makes it impractical for clinical use for induction of pharmacological preconditioning,” noted the research team. The team introduced calorie restriction to the mice as an alternative intervention.
Feeding the mice 40% less food for six weeks led to a significant reduction in myocardial infarction size with an upregulation of Nampt compared to mice with a normal diet. However, the mechanism of calorie restriction is complex and needs future investigation.
Injecting NMN and calorie restriction both upregulates Nampt and protects the heart by mimicking the effects of ischemic preconditioning. The next step for the scientists is to understand the Nampt regulating molecular mechanism, which may allow them to target the molecule and develop interventions to protect the heart against I/R injury.

