NMN Inhibits COVID-19 Infection, According to Chinese Study
Age-associated DNA damage promotes viral entry of SARS-CoV-2 into the lung cells of mice, yet NMN prevents this infection.
Highlights
- SARS-CoV-2 viral entry is higher in human lung cells treated with DNA damage-inducing radiation.
- Inhibiting DNA repair hinders cell surface accumulation of primary receptors for SARS-CoV-2 (ACE2 receptors), suggesting a link between DNA repair and receptor-mediated viral entry.
- NMN, known to help repair DNA, dampens cell surface ACE2 receptor buildup along with SARS-CoV-2 viral load in mice.
A peculiar phenomenon associated with SARS-CoV-2, the virus that causes the COVID-19 infection, is that it affects older adults at much higher rates than younger ones. According to the Center for Disease Control, hospitalization rates are 5-10 times higher in adults over age 65 compared to younger counterparts aged 18-29 years. An inherent aspect of aging which may predispose older adults to COVID-19 infections is the accumulation of DNA damage. However, a link between age-related DNA damage and COVID-19 infections had not been firmly established.
Published in Aging Cell, Cheng and colleagues from the Beijing Institute of Biotechnology found higher SARS-CoV-2 viral entry into human lung cells with radiation-induced DNA damage. Additionally, inhibiting the DNA damage response (DDR), the cell’s DNA repair mechanism, inhibits the accumulation of receptors that SARS-CoV-2 uses for cell entry – angiotensin-converting enzyme 2 (ACE2) receptors. Treating mice with 200 mg/kg/day of nicotinamide mononucleotide (NMN) in drinking water preserved DNA, prevented ACE2 receptor accumulation, and reduced the viral load in the lungs after infection. These findings suggest that age-associated DNA damage and the cell’s DNA repair mechanisms trigger ACE2 receptor accumulation and viral infection but that NMN can counter this means of infection.
DNA Damage Induces Viral Entry into Human Cells
To find out how DNA damage influences SARS-CoV-2 entry into cells, Cheng and colleagues used human lung cells exposed to DNA damage-inducing ionizing radiation. Following seven days of exposure to the radiation, the cells absorbed approximately six times more of the virus than cells not exposed to radiation. Moreover, inhibiting the cells’ DNA repair response significantly reduced viral entry into the cells. These findings provide evidence that DNA damage associated with radiation triggers SARS-CoV-2 viral entry into cells but that inhibiting the DDR reduces this viral entry.

Cheng and colleagues went on to examine the radiation’s effects on the primary receptors that SARS-CoV-2 uses for cellular entry – ACE2 receptors. The Beijing-based team found that exposure to ionizing radiation increased ACE2 receptor levels six-fold, however, inhibiting the DDR impaired ACE2 buildup. These results suggest that DNA damage and the DDR trigger an upsurge of cell surface ACE2, which correlates with increased viral entry into cells.
NMN Prevents Viral Entry into Infected Mouse Lung Cells
Enzymes with roles in DNA repair, such as sirtuins and poly (ADP-ribose) polymerases (PARPs), require the essential nicotinamide adenine dinucleotide (NAD+) molecule to function. For this reason, Cheng and colleagues supplemented old mice exposed to a mouse version of SARS-CoV-2 with the NAD+ booster NMN. They found that NMN treatment correlated to lower DNA damage and ACE2 receptor levels along with reduced lung viral loads. These results demonstrate that, at least in mice, NMN wards off SARS-CoV-2 infection, possibly by maintaining DNA and blocking the buildup of ACE2 receptors.
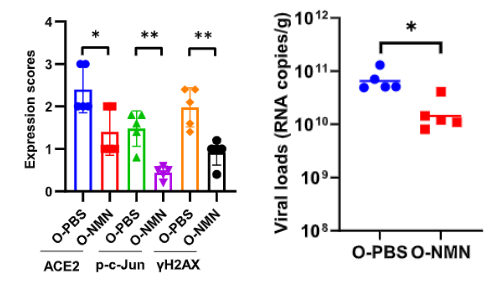
NMN May Preserve DNA and Hinder ACE2 Receptor Accumulation
“Here, we identified that targeting DNA damage … by increasing the DNA repair capacity through treatment with NMN, reduced the expression of ACE2,” said Cheng and colleagues. “More importantly, … NMN reduced viral loads.”
Overall, this study suggests that NMN may prevent the accumulation of ACE2 receptors in the lung, kidney, and intestines of older individuals to prevent excessive viral entry of SARS-CoV-2. Theoretically, this NAD+ booster may prevent the accumulation of DNA damage by stimulating sirtuins and PARPs to maintain DNA, thus dampening the activation of the DDR and reducing ACE2 receptor buildup.
Model: C57BL/6J mice
Dosage: 200 mg/kg/day of NMN in drinking water

