NMN Protects Brain Cell Survival and Cognitive Function From Severe Hypoglycemia
Hypoglycemia, severely low blood sugar, a common compilation in diabetic patients, can lead to brain injuries. But, NMN may ameliorate neuronal damage and cognitive impairment that follows the condition.
The high-caloric, inactive modern lifestyle has pushed our metabolism beyond what it can handle. Over 34 million Americans have diabetes and 90 percent of those have type 2 diabetes, in which the body either resists the effects of insulin, sugar-regulating hormones, or doesn’t produce enough to maintain normal blood sugar levels.
Now, Chinese researchers found that nicotinamide mononucleotide (NMN) can improve neuronal survival and cognitive functions in rats with hypoglycemia-induced brain injury. The study, published in Brain Research Bulletin, suggested that NMN may be a new intervention to prevent it.
Hypoglycemia eats away the brain
For diabetes patients, one of the treatments is insulin therapy, in which patients get insulin shots to bring down blood sugar. Although controlling blood sugar levels reduced the risk of diabetes compilation, but it also increased the risk of severe hypoglycemia, also known as low blood sugar that may induce serious brain injury.
Clinically, the first treatment for severe hypoglycemia patients is to raise blood sugar levels. But, the reperfusion of blood sugar causes a secondary injury, inducing severe neuronal death. While healthy rat neurons in the hippocampus, the learning and memory center in the brain, appear round with a clear and intact structure, neurons in rats with severe hypoglycemia showed degenerated neurons with a significant loss in numbers.
NMN protects the brain against hypoglycemia
However, the researchers found that NMN can reduce neuronal death after severe hypoglycemia. Diseased rats that received glucose and NMN injection treatment had 83 percent less neuronal death than those that only had glucose. When the researchers gave rats a chemical that generated inactive nicotinamide adenine dinucleotide (NAD+), the downstream molecule of NMN, neuronal death increased by 449 percent, reversing NMN’s protective effects. The results indicated the vital role that NAD+ plays in preventing neuronal death.
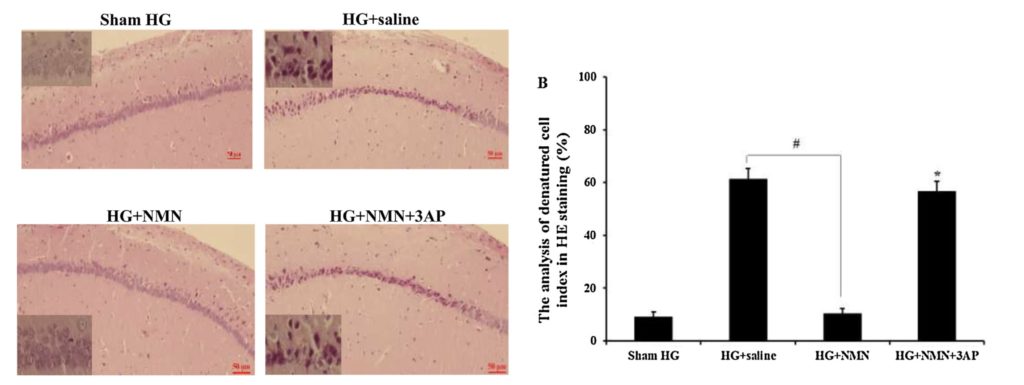
(Wang et al., 2020) NMN administration reduced neuronal death after severe hypoglycemia. The right figure show hippocampal neuron staining of normal rats (Sham HG), hypoglycemia rats with saline (HG + saline), hypoglycemia rats with NMN (HG + NMN), and hypoglycemia rats with NMN and chemical that generated inactive NAD+ (HG + NMN + 3AP). The left figure shows the number of denatured cells.
Hypoglycemia leads to an accumulation of reactive oxygen species (ROS), unstable molecules that may cause damage to the DNA in cells. In response to the DNA damage, the cell activates a repair pathway that depletes energy molecules and relies heavily on NAD+, NMN’s downstream molecule, and an essential molecule for molecular reactions. The loss of energy molecules and NAD+ often results in neuronal death and cognitive impairment.
In rats with severe hypoglycemia, NMN treatment not only reduced ROS formation and the activation of the molecular repair pathway but also restored both the levels of NAD+ and energy molecule. Moreover, the NMN administration also prevented spatial learning and memory deficits after severe hypoglycemia.
Six weeks after the hypoglycemia event, the researchers introduced the rats to a water maze, where the faster the animals escape the maze, the better their cognitive performance is. Animals treated with NMN escaped the maze more quickly than the untreated or those that were treated by NMN and NAD+-inactivating chemicals.
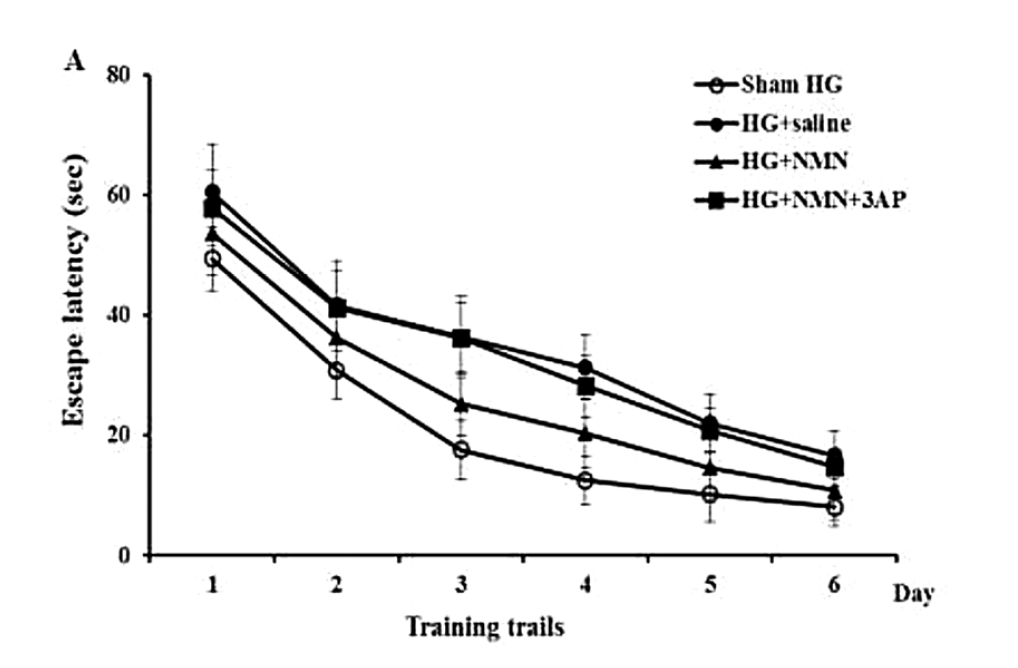
(Wang et al., 2020) NMN administration prevented severe hypoglycemia-induced cognitive impairment. Rats treated with NMN showed lower escape latencies, meaning that the animals escaped the maze faster.
“Taking together, NMN protects the brain against severe hypoglycemia-induced neuronal damage and its associated cognitive deficits,” writes the authors. The researchers also noted that administering NMN after hypoglycemia events in the study mimics real-world conditions where doctors will treat the patients with glucose and a potential neuroprotective drug in the emergency room. “Our study suggests that NMN may be an effective intervention for patients with severe hypoglycemia.”

