NMN Generating Enzyme Drives Fasting-Related Fat Burning
Researchers from the Washington University School of Medicine in St. Louis show that the enzyme behind NMN biosynthesis – NAMPT – reverses metabolic derangements in obese mice.
Highlights
- NAMPT, the enzyme that generates NMN, is needed to enhance the balance of fat (lipid) and sugar (glucose) in metabolically stressed mice.
- In diabetic mice, NAMPT increases thermogenesis – the metabolic process by which organisms burn calories to generate heat.
- These data highlight a direct therapeutic role for NAMPT activation and NMN administration in liver cells to mimic the effects of fasting.
Intermittent fasting and caloric restriction improve metabolism and inflammation in mice and humans. That’s why both intermittent fasting and caloric restriction have been pursued as promising means by which to abate diseases of aging, overnutrition, and neurodegeneration. Calorie restriction abates aging and cardiometabolic disease by activating metabolic signaling pathways, including the nicotinamide adenine dinucleotide (NAD+) synthesis pathway.
Published in Nature Communications, researchers from Washington University School of Medicine in St. Louis show that NAMPT – an enzyme necessary for synthesizing the NAD+ precursor NMN – in the liver drives critical aspects of the fasting response. On the one hand, mice lacking NAMPT in liver cells exhibit defective thermal regulation during fasting and are sensitized to diet-induced glucose intolerance. On the other hand, increasing levels of NAMPT in liver cells induces fat browning, improved glucose balance, and attenuated high blood fat levels (dyslipidemia) in obese mice. This work shows that modulating NAD+ levels in liver cells can potentially mitigate fasting-associated disease.
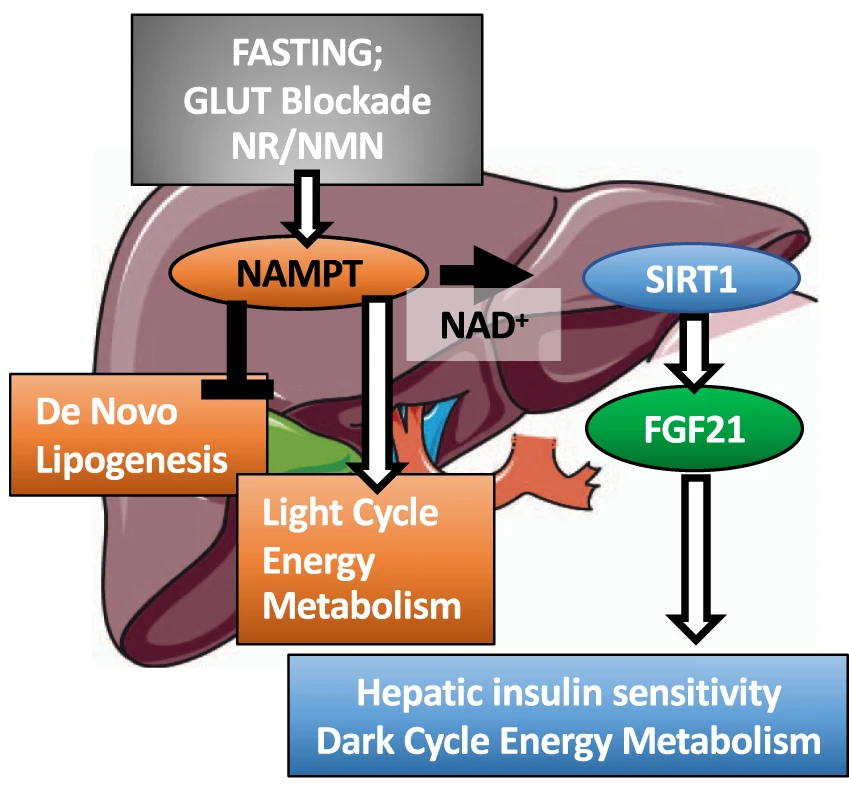
(Higgins et al., 2022 | Nature Communications) NAMPT in the liver balances energy metabolism. Liver cell NAMPT activation promotes light- and dark-cycle energy metabolism and liver insulin sensitivity while blocking the formation of fat (de novo lipogenesis). NAMPT can be activated by blocking glucose transporters (GLUT) or through supplementation with the NAD+ precursors nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN). NAMPT = nicotinamide phosphoribosyltransferase, SIRT1 = sirtuin 1, FGF21=fibroblast growth factor 21.
The Liver Is Central to Metabolic Regulation
The liver is uniquely positioned at the nexus between blood coming from the digestive system and blood being sent back to the heart to be pumped to the brain, limbs, and the rest of the body. Here, the liver can sense, coordinate, and transition between different states of metabolism that depend on feeding or the lack thereof (also known as fasting). Nutrient withdrawal creates an adapt-or-perish proposition for an organism in general and for liver cells in particular. The lack of sugar forces a reliance upon fat breakdown throughout the body, which gets metabolized in the liver. When this occurs, the liver needs to make several key adjustments. NAD+ synthesis unifies the adaptive responses to each of these stresses in the nutrient-restricted liver. Accordingly, NAD+ protects against diseases ranging from aging and neurodegeneration to diabetes and non-alcoholic fatty liver disease (NAFLD).
Liver NAMPT Is Necessary for Fasting Benefits
In this study, the Washington University of Medicine in St. Louis researchers dissect how NAMPT in liver cells modulates metabolic balance. Higgins and colleagues demonstrate that liver NAMPT mediates a key liver signaling cascade during fasting. The study shows that fasting and glucose transport inhibition upregulates NAMPT in the liver, while liver-specific NAMPT deficiency impairs several key fasting metabolic processes. More specifically, mice lacking NAMPT in the liver show defects in glucose metabolism, whereas viral-mediated increases in liver NAMPT enhance thermogenesis and glucose metabolism in diet-induced and genetically obese models. These findings show that liver NAMPT levels are essential to protect against diet-induced glucose intolerance and to elicit the metabolic benefits of fasting.
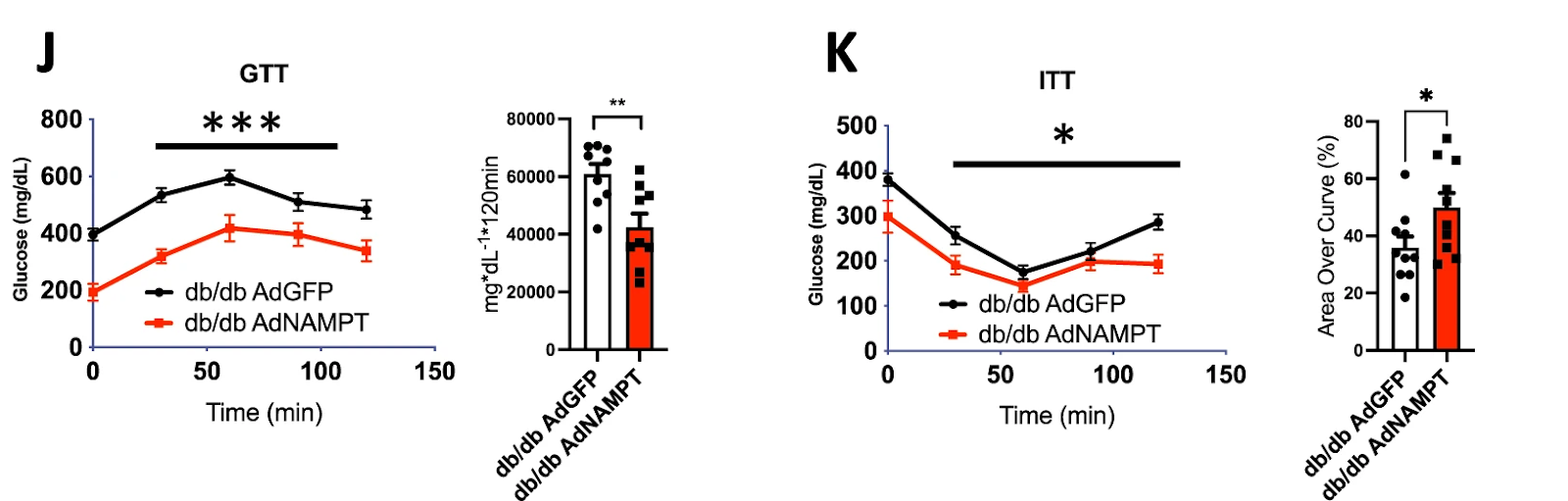
(Higgins et al., 2022 | Nature Communications) Liver NAMPT enhances glucose homeostasis in diabetic mice. Increased levels of NAMPT in obese, diabetic mice (db/db AdNAMPT) led to lower plasma glucose throughout both glucose and insulin tolerance testing, indicating improved glucose metabolism and insulin sensitivity.
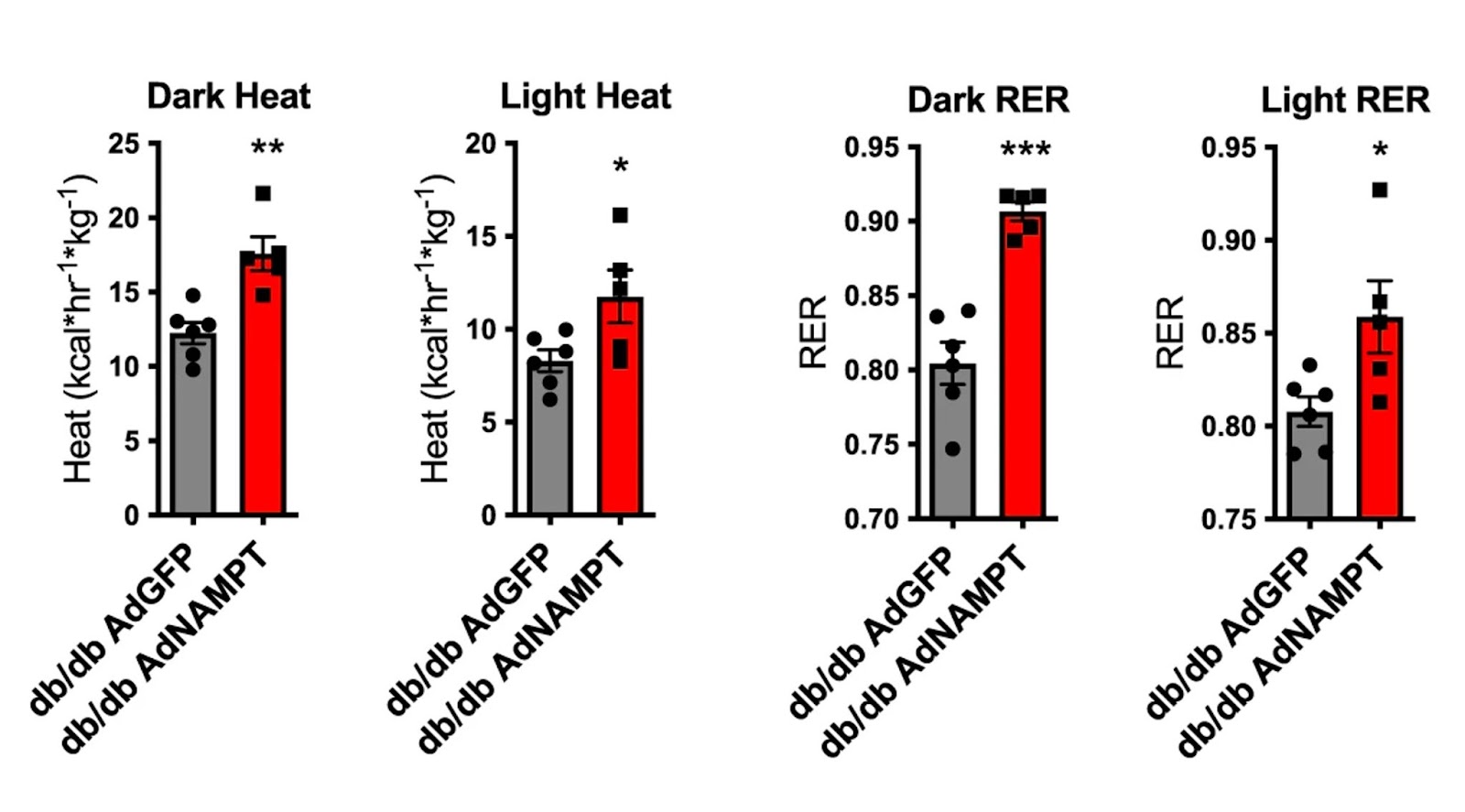
Higgins and colleagues found that raising NAMPT levels in obese diabetic mice increased fat tissue browning markers. Fat tissue (usually white) turns brown when it is thermogenically active, meaning that it generates more heat while burning calories. Along these lines, mice with elevated liver NAMPT had higher thermogenesis levels than controls. Raising liver cell NAMPT induced increased heat production and gas exchange – more oxygen in and carbon dioxide out during breathing – in mice during both light- and dark cycles, whereas NAMPT deletion led to dark-cycle thermic defects. The data indicate that liver cell NAMPT signaling distinguishes between light- and dark-cycles when modulating energetic control.
Higgins and colleagues conclude that NAMPT in the liver exerts broad fasting-mimetic effects downstream of generalized fasting and glucose transport inhibition in liver cells. This study suggests that metabolic disease can be intervened at the level of NAD+ biosynthesis via NMN. It is possible that NAMPT activation in the liver can fight against aging, obesity, and other fasting-responsive diseases. Interestingly, there are some compounds that activate NAMPT like SBI-797812, which turns NAMPT into a “super catalyst” that more efficiently generates NMN. Of note, mice dosed with SBI-797812 show elevation in liver NAD+. For all we know, this could be the basis for that long-sought-after miracle fasting pill.

