Research Shows NMN Supports Mitochondrial Replication
Just like the genomes in the nuclei of our cells, these energy-generating structures have their own set of DNA, which is a proxy measure for mitochondrial function and has been associated with several aging-related diseases.
Highlights
- Japanese researchers examine the role of nicotinamide mononucleotide (NMN) on human mitochondria.
- Boosting nicotinamide adenine dinucleotide (NAD+) levels with NMN may help deter aging by increasing mitochondrial metabolism.
Our cells contain more than one set of DNA: one in our nucleus encodes most cellular processes and another in the energy-generating structure of the cell known as the mitochondria. Just like the DNA in the nuclei of every cell, the replication and integrity of mitochondrial DNA (mtDNA) is critical for cells to function and flourish and for us to fulfill a healthy and long life. But we have a lot to learn about the link between mtDNA and mitochondrial function in the context of aging and longevity. Doing so can enable us to bolster the activity of this crucial cell structure and integrity of its separate genetic hard drive for improving healthspan and lifespan.
Kang and colleagues from Kyushu University in Japan reported in the Journal of Biochemistry that nicotinamide mononucleotide (NMN) enhances mtDNA replication. The researchers, who wrote on behalf of the Japanese Biochemical Society, found that treating human kidney cells with NMN activated and increased the rate of mtDNA replication by increasing the number of building blocks of DNA (nucleotides) in mitochondria while decreasing their degradation products (nucleosides). These findings suggest a mechanism for how NMN benefits mitochondria, metabolism, and, ultimately, aging.
The Role of NAD+ in mtDNA Replication
In cooperation with many nuclear-encoded proteins, mtDNA genes contain the instructions to components for essential cell processes, such as generating energy. Mitochondrial DNA copy number, a measure of the number of mitochondrial genomes per cell, is a minimally invasive proxy for mitochondrial function and has been associated with several aging-related diseases and all-cause mortality. However, we still only know a little about how metabolism inside mitochondria affects mtDNA maintenance and replication, let alone how these processes may underlie aging and longevity.
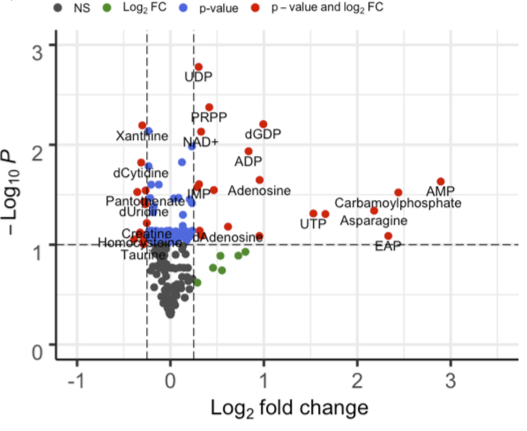
To address this problem, Kang and colleagues created a new method for measuring metabolism within mitochondria to understand how mitochondrial metabolism regulates mtDNA replication. With this method, which they called SLO, The Japanese research team genetically manipulated levels of an enzyme called ‘twinkle’ — a component of mtDNA replication machinery that can increase the number of mitochondria.
By increasing mtDNA copy number through twinkle manipulation, Kang and colleagues found that several components related to metabolism changed, including increases in nucleotides and NAD+ — a vital molecule that serves as an essential factor for innumerable cell processes, including mitochondrial energy generation. Kang and colleagues speculate that these results suggest that nucleotide and NAD+ increases may result from increased demand for components required for mtDNA replication.
NMN Modulates Mitochondrial Replication
Kang and colleagues then examined the nature of the relationship between mtDNA replication and NAD+. To show that NAD+ causally influenced mtDNA and was not just correlative, the researchers treated human kidney cells with NMN, a precursor of NAD+, for three consecutive days and then quantified the amount of mtDNA. In NMN treated cells, the amount of mtDNA increased to levels comparable to a condition activating mtDNA replication by controlling the ‘twinkle’ protein that executes this mtDNA copying process. After establishing that NMN administration can increase the amount of mtDNA, Kang and colleagues showed that NMN also increases the rate of mtDNA replication. Combining NMN administration and twinkly activation enhanced the beneficial effects on mtDNA replication and copy number.
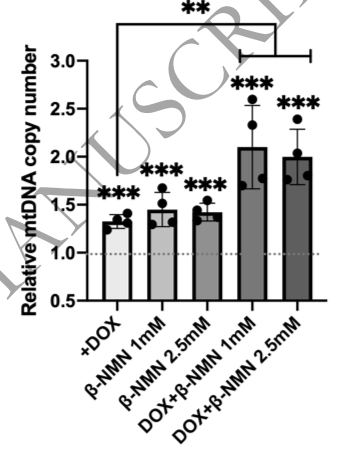
(Kang et al., 2021 | Journal of Biochemistry) NMN enhances mtDNA replication. Kang and colleagues compared the amount of mitochondrial DNA (mtDNA copy number) in conditions where the mtDNA replication protein twinkle was activated (DOX), NMN was administered, and the combination of these two. These results show that relative to untreated cells, all conditions show increases in mtDNA number. The actions of twinkle activation together with NMN have a combined effect.
Kang and colleagues conclude the article by discussing how these data show that NMN and mtDNA replication are functionally linked through the regulation of nucleotide pool synthesis. They indicate that they are further investigating this issue to clarify the underlying mechanism.

