NMN Eases Neurological Damage Following Traumatic Brain Injury: China Study Confirms
Nicotinamide mononucleotide (NMN) alleviates cognitive dysfunction by minimizing neurological damage and inflammation following traumatic brian injury (TBI) in rats.
Highlights
- NMN treatment substantially improves learning and memory in rats with TBI.
- NMN curbs neurological damage from TBI in rats.
- NMN treatment alleviates the brain’s gene activity signatures indicative of inflammation following TBI.
Brain injury is one of the global leading causes of adult disability, with a yearly incidence of about 50 million adults worldwide. The initial impact of a TBI precipitates damage, however, the secondary injuries that occur in the minutes, hours, and days following a TBI are the most severe. Secondary injuries result from complex pathological reactions, including neuronal cell death and inflammation, causing irreversible brain damage. While treatments like omega-3 fatty acids can reduce neuronal death by diminishing inflammation, more effective remedies are needed to alleviate damage from secondary injuries.
Published in the International Journal of Medical Sciences, Zhao and colleagues from Wuhan University in China show that NMN prevents spatial learning and memory impairments following TBI in rats. NMN eases neurological damage after TBI, providing a likely explanation for the preservation of learning and memory. NMN also curbs the increased gene activity of inflammatory proteins that coincide with inflammation from TBI. These findings suggest that taking NMN may be a way to mitigate the cognitive dysfunction and neurological damage that go with TBI.
“Our data might provide a novel therapeutic strategy for TBI,” said Zhao and colleagues.
NMN Preserves Cognition, Prevents Neurological Damage, and Reduces Inflammation
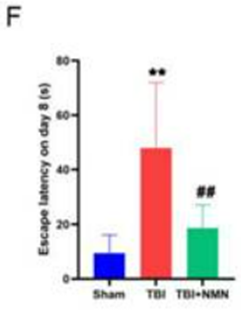
Zhao and colleagues used rats that received a series of controlled cranial impacts to induce the TBI. To test NMN’s effects on TBI, the researchers injected the rats with a 43.75 mg/kg single dosage of NMN one hour after inducing TBI. The rats were tested with the Morris water maze – a measurement of spatial learning and memory – by examining the amount of time it took to find an escape platform in a cylinder of water. Following TBI, non-treated rats took about five times longer to find the escape platform, however, the NMN-treated rats with TBI took much less time to find the escape platform. With lower times to find the escape platform comparable to rats that didn’t undergo TBI, NMN-treated rats with TBI showed preserved spatial learning and memory.
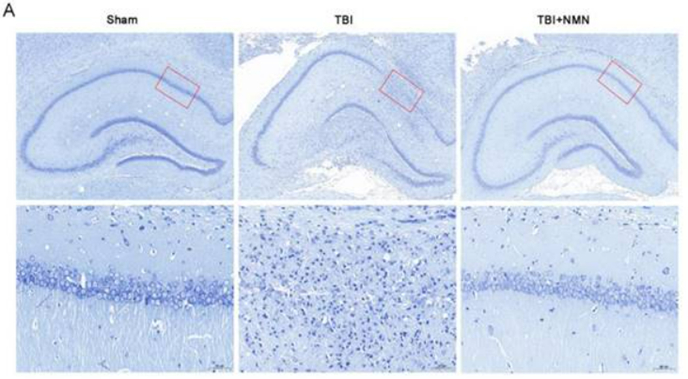
In search of an explanation for the learning and memory improvements, Zhao and colleagues examined neurons in a brain region associated with learning and memory – the hippocampus. They found that neurons in this region were less abundant and more disorderly following TBI, but NMN restored their numbers and induced a more structured organization. These findings suggest NMN mitigates neurological damage following TBI.
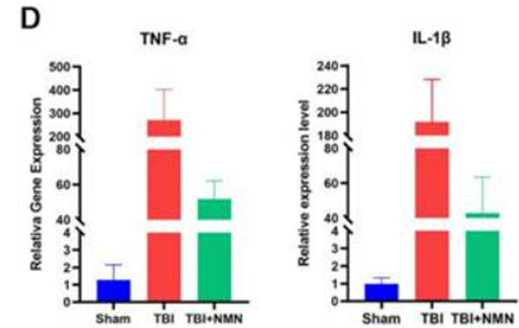
Because inflammation can lead to neuronal death and neurological damage, Zhao and colleagues analyzed the gene activity of two proteins associated with inflammation – TNF-α and IL-1ꞵ. For the former, gene activation increased ~300-fold, while the latter increased ~200-fold with TBI. For both proteins, NMN decreased the gene activity elevation five-fold with TBI, suggesting less inflammation with NMN.
NMN Could Be a Novel Therapeutic for TBI
Zhao and colleagues present the first study applying NMN to treat TBI, with results suggesting NMN preserves cognition and alleviates neurological damage. Previous research has shown that stimulating NMN production alleviates TBI in mice, but this is the first TBI study in rodents that supplemented with NMN directly. Other studies have already shown that supplementing NMN to increase levels of the essential molecule for DNA repair and cell energy production – nicotinamide adenine dinucleotide (NAD+) – improves metabolism and reduces inflammation. With their study, Zhao and colleagues’ data provide evidence for one more condition that NMN can counter – severe brain injury.
Model: Sprague-Dawley rats
Dosage: 43.75 mg/kg single intraperitoneal injection one hour after TBI

