NMN Attenuates Fat Tissue Inflammation and Scarring, New Study Shows
Nicotinamide mononucleotide (NMN) alleviates fat tissue inflammation and scarring mediated by low tissue oxygen (hypoxia) – similar to obesity conditions – in mice.
Highlights
- NMN treatments curtail harmful oxygen deprivation-induced fat tissue scarring (fibrosis) in mice.
- NMN alleviates fibrosis by reducing inflammatory proteins, and increasing levels of an anti-inflammatory and pro-insulin sensitivity protein called adiponectin.
- The treatments lessen the abundance of pro-fibrosis and inflammation-triggering protein hypoxia-inducible factor-1ɑ (HIF-1ɑ).
Low-grade inflammation driving metabolic conditions like type 2 diabetes and cardiovascular diseases is a key symptom of obesity. This state of inflammation triggers fat tissue fibrosis that volumetrically expands more than normally-functioning fat tissue, leading to oxygen deprivation (hypoxia) from lack of blood flow. Hypoxia drives further inflammation and fat tissue fibrosis, creating a snowball effect where defective fat tissue perpetuates more damaged and harmful fat tissue. Finding a way to counter this spread of fat tissue damage has become paramount in researchers’ quest to mitigate the obesity epidemic.
Published in Frontiers in Endocrinology, Liu and colleagues from Central South University in China show that NMN injections counter fibrosis in mice with hypoxia-induced fat tissue fibrosis. The researchers show that NMN reduces levels of inflammatory proteins and increases the abundance of pro-insulin sensitivity and anti-inflammatory protein adiponectin. They go on to show that hypoxia increases levels of a protein that initiates fat tissue inflammation and fibrosis – HIF-1ɑ – but that NMN attenuates its abundance. Findings from the study suggest NMN can ward off the snowball effect where fibrotic fat tissue begets more dysfunctional fat tissue in obesity.
NMN Attenuates Fat Tissue Fibrosis by Reducing HIF-1ɑ Protein Levels
To induce the dysfunctional inflammatory and fibrotic state of fat tissue, Liu and colleagues placed mice in a chamber with low oxygen – hypoxia – for four weeks. They found that hypoxia increased fibrosis about four-fold as measured by the quantity of scar tissue proteins, namely collagen. NMN treatments more than cut in half the hypoxia-driven fibrosis in fat tissue, suggesting that NMN may provide a means to treat fat tissue fibrosis and dysfunction.
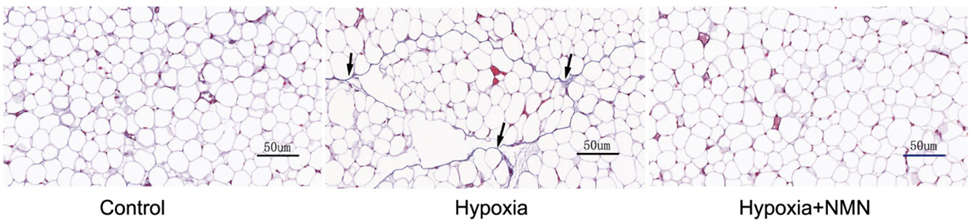
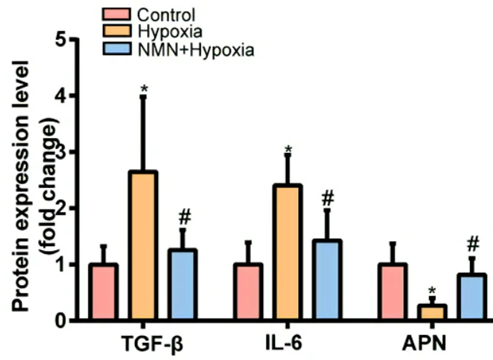
Since inflammation is one of the factors contributing to fibrosis, the China-based research team tested whether NMN reduces hypoxia-induced fibrosis by lowering inflammatory proteins and increasing anti-inflammatory proteins. Along those lines, they measured levels of the inflammatory molecules TGF-𝛃 and IL-6, along with the pro-insulin sensitivity and anti-inflammatory protein adiponectin. As expected hypoxia more than doubled levels of the two inflammatory proteins and more than cut in half adiponectin. However, NMN substantially reduced the hypoxia-induced inflammatory protein elevations and significantly increased adiponectin levels after hypoxia. These results suggest that NMN attenuates hypoxia-driven fibrosis by reducing inflammatory proteins and increasing adiponectin.
Since the protein HIF-1ɑ initiates and drives hypoxia-induced fat tissue fibrosis, Liu and colleagues measured the protein’s levels with hypoxia. Hypoxia more than doubled HIF-1ɑ levels, but NMN treatments countered the elevated HIF-1ɑ proteins. These findings suggest that NMN reduces HIF-1ɑ levels under hypoxic conditions to reduce inflammation and fibrosis.
“Our study showed that NMN inhibited HIF-1ɑ activation-induced adipose tissue fibrosis and inflammation,” said Liu and colleagues.
Potential New Way to Treat Obesity
With 41.9% of the US population being obese, new treatment strategies to counter fat tissue inflammation, fibrosis, and its subsequent dysfunctional proliferation are paramount. Previous research has shown that NMN counters kidney, liver, and cardiac fibrosis, yet this is the first study to suggest that it can attenuate fat tissue fibrosis.
With recent research from Harvard showing that NMN helps with weight loss, this study illustrates one of the ways that NMN could confer its anti-obesity effects. The study suggests that by inhibiting HIF-1ɑ protein buildup, NMN can thwart fat tissue inflammation and fibrosis, thus inhibiting the perpetual accumulation of dysfunctional fat tissue.
Model: C57/B6 mice
Dosage: 500 mg/kg injected intraperitoneally every three days up to five days before hypoxia and then once after hypoxia

