NMN Alleviates Kidney Tissue Scarring in Rodents
Stimulating a longevity-linked protein with nicotinamide mononucleotide (NMN) reduces tissue scarring progression and subsequent kidney disease.
Highlights
· Rodents lacking SIRT1 have an aggravated kidney scarring response.
· Stimulating SIRT1 in rodent kidney cells with NMN reduces the activation of the pro-scarring markers.
When our kidneys become diseased, they undergo irreversible changes in function and overall structure that progress over months and years. Tissue scarring, or what’s called fibrosis in the medical field, contributes substantially to the advancement of kidney disease. We currently lack treatment options to delay and prevent kidney disease complications and progression from tissue scarring. So, understanding how tissue scarring progresses has become urgent for developing better therapeutic options.
Huang and colleagues from Nantong University in China published a study in Cell Death Discovery indicating that stimulating a protein linked to anti-aging that is involved in metabolism called sirtuin1 (SIRT1) attenuates kidney scarring progression in mice. After causing kidney damage, the research team saw that boosting levels of nicotinamide adenine dinucleotide (NAD+), a factor essential for SIRT1 activity, diminished tissue scarring markers. These findings provide some illumination for the cellular mechanisms by which tissue scarring accumulates to facilitate kidney damage, which may help with the development of therapeutic options to slow age-related kidney disease.
Boosting NAD+ to Activate SIRT1
Sirtuin proteins like SIRT1 play essential roles in metabolism, DNA repair, and aging processes and depend on the crucial bioenergetic molecule NAD+ to function. NAD+ levels can be boosted by supplementing different precursor molecules, such as nicotinamide mononucleotide (NMN), in mammals like rodents and possibly humans. Also, the levels of NAD+ in cells decline throughout the body, and most likely in kidney cells, with age in various species including humans, and whether age-associated kidney scarring can be mitigated by increasing NAD+ levels with NMN to activate SIRT1 has not been thoroughly explored.
SIRT1 Deficiency Aggravates Kidney Scarring
To find out what effect the SIRT1 protein plays in kidney scarring, Huang and colleagues genetically eliminated SIRT1 function in mice and induced kidney scarring. They found that getting rid of SIRT1 aggravated kidney damage from the scarring. Their findings showed that a SIRT1 deficiency worsens kidney scarring and suggests that SIRT1 plays a protective role against kidney tissue scarring.
Kidney Scarring Depends on HIF-2⍺
Huang and colleagues then shifted their focus to rat kidney cells to catch a closer glimpse of how SIRT1 is involved in preventing kidney fibrosis. When the research team chemically-induced kidney scarring, they found that the levels of the HIF-2α protein, which helps cells survive under low oxygen conditions, increased in kidney scarring. Interestingly, when they genetically deleted HIF-2α from mice and tried to induce kidney scarring, they saw a reduced level of kidney damage.
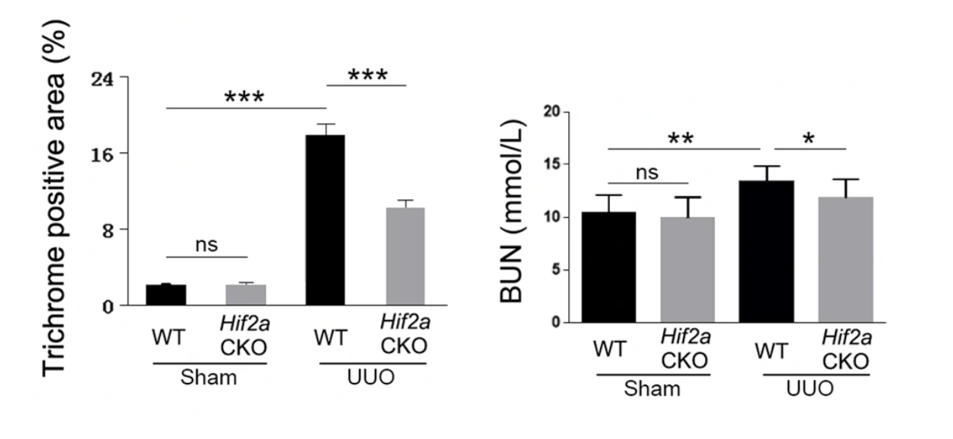
NMN Stimulates SIRT1 to Reduce Kidney Scarring Mediated by HIF-2⍺
When the researchers added NMN to stimulate SIRT1 activity, they saw decreased HIF-2α levels, suggesting that SIRT1 represses HIF-2α protein activity. To support this, when Huang and colleagues added the SIRT1 suppressor sirtinol, they saw increased HIF-2α protein levels along with increased protein markers of scarring. These findings suggest that the kidney tissue-protective roles of SIRT1 come from HIF-2α protein suppression.
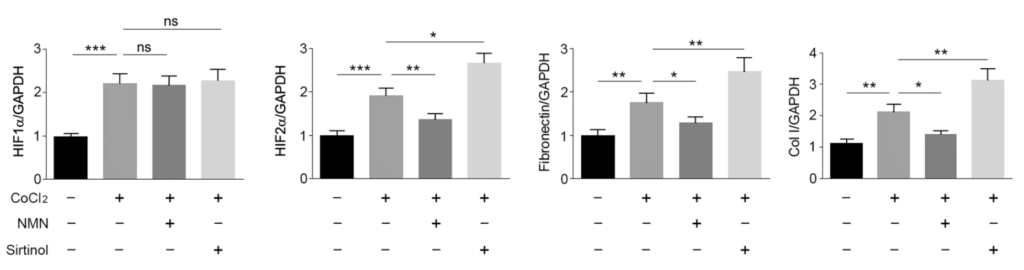
“Together, our data indicate that SIRT1 plays a protective role in renal damage and fibrosis, which is likely due to inhibition of HIF-2α,” said Huang and colleagues. They go on to propose that the SIRT1/HIF-2α axis may provide a new target for the treatment of renal fibrosis and chronic kidney disease.
Can NMN Prevent Kidney Scarring in Humans?
Applying this pathway information may help to develop new therapeutic agents to prevent or slow the progression of age-related kidney scarring. It also suggests that increasing NAD+ levels with NMN to stimulate SIRT1 may already offer a means to slow kidney scarring. Determining whether NMN can slow kidney disease progression in humans as it does in rodents will require future clinical trials.

