New Treatment Selectively Destroys Aging-Linked Cells
Researchers have fused antibodies with a cell toxic compound to remove non-proliferating (senescent) cells without harming non-senescent cells.
Highlights
· Antibodies targeting the cell surface protein B2M, present only on senescent cells, were linked to a cell toxic compound to specifically destroy senescent cells.
· The antibody-drug conjugate primarily eliminates cells that enter senescence from stress instead of excessive divisions (replicative senescence).
· Joining antibodies specifically targeting senescent cells to toxic drugs could be an efficient way to selectively kill senescent cells and prevent age-related tissue decline.
As we grow older, our organs increasingly accrue non-proliferating (senescent) cells. Although senescent cells are present in all tissues throughout our lifetimes, their abundance crosses a threshold during aging where the inflammatory proteins they release become harmful. This senescent cell accumulation and release of damaging molecules influence the onset of age-related diseases like diabetes, cancer, and Alzheimer’s. So, finding ways to counteract the age-associated buildup of senescent cells by targeting and eliminating them could help preserve tissue health and possibly prevent serious age-related diseases.
Macip and colleagues from the University of Leicester in England published in Scientific Reports showing that they linked a toxic compound with an antibody that recognizes senescent cells. The antibody-drug fusion aimed at senescent cells primarily destroyed the cells entering a stress-induced non-proliferating state. Eliminating senescent cells offers hope for diminishing their presence during aging and alleviating age-related diseases.
“Just like our antibodies recognize germs and protect us from them, we’ve designed these antibodies to recognize old cells,” said the study’s lead author Macip in a press release. “In addition, we’ve given them a toxic load to destroy them, as if they were a remote-controlled missile.”
Senescent Cells Have A Unique Surface Protein
Macip and colleagues had the idea that they could deliver toxic drugs into senescent cells if they could identify proteins specific to senescent cells exposed on the surface. Once identified, they could then generate antibodies that recognize these proteins and therefore target only senescent cells. The Leicester-based team had previously identified the protein molecule B2M as a cell surface protein marker for senescent cells. So, they began their study by examining this protein and found that its abundance progressively increased with age in skin and brain tissues in mice, suggesting its presence on aged senescent cells.
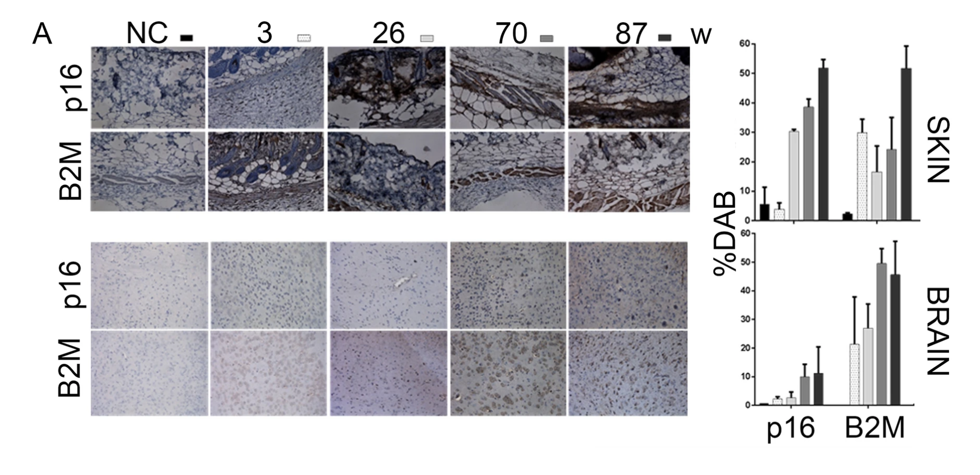
To then confirm B2M’s localization to the surface of senescent cells, Macip and colleagues used human bladder cancer cells in laboratory dishes to induce senescence. They genetically engineered these cancer cells to overexpress one of three senescence-promoting proteins: p53, p21, or p16. They found that generating excessive levels of p53 — an inducer of senescence from stress — was significantly linked to higher B2M levels. So, these findings suggest that B2M levels increase on senescent cells triggered by p53 stress-induced senescence.
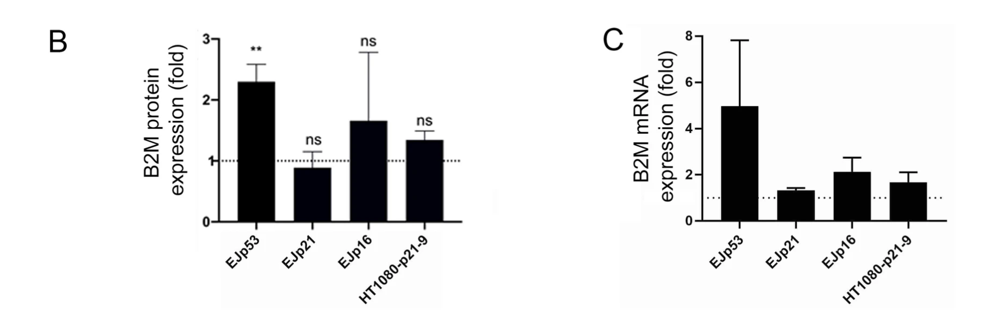
A B2M-Targeting Antibody Linked to Duocarmycin Kills Senescent Cells
After confirming the presence of B2M on senescent cells, Macip and colleagues designed an antibody specific to this protein linked to duocarmycin — a compound toxic to cells. Linking duocarmycin to a B2M-specific antibody should then theoretically destroy only senescent cells. Indeed, in senescent cells induced by the p53 protein (stress-induced senescence), but not in p16 induced senescent cells (those with replicative senescence), the antibody-drug conjugate destroyed senescent cells. Treating cells with the antibody-drug conjugate targeting B2M left non-senescent cells intact, providing evidence that the antibody-drug conjugate specifically targets senescent cells.
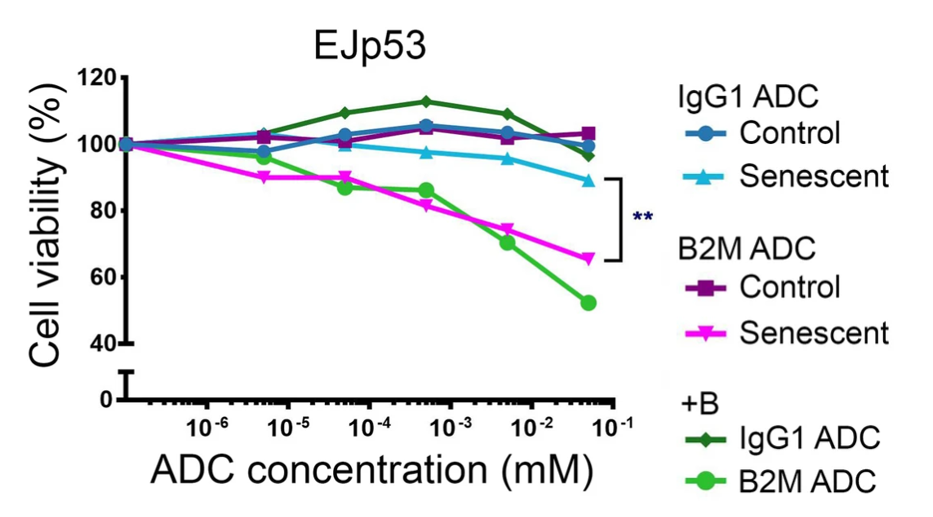
Can Humans Use an Antibody-Drug Conjugate?
The study results show that the antibody-drug conjugate against the B2M surface protein can eliminate senescent cells, at least in laboratory dishes. Primarily, senescent cells with stress-induced senescence were destroyed, not replication-induced senescence, suggesting possibilities for other techniques targeting varieties of senescence. To achieve an antibody-drug fusion that targets replicative senescent cells, researchers will need to pin down and target surface proteins that result from p16 protein-induced replicative senescence.
Since the B2M surface protein shows up across in most tissues, similar antibody-drug conjugates could target other surface proteins present in specific tissues. Tissue specificity could be important because destroying senescent cells may have different effects in certain tissues compared to others. In essence, we don’t yet know the long-term effects of eliminating senescent cells across an array of tissues on overall health.
“Our results indicate that antibodies could be an efficient system to bring toxic drugs into specific types of senescent cells in humans with minimal side effects,” said Macip and colleagues in their publication.
One major limitation of this study is that the targeting of senescent cells was performed in laboratory dishes. In many situations, the results from lab cultured cells haven’t translated to whole organisms like mice, let alone humans. So, we’re still likely far away from using this kind of senescent cell killer (senolytic) agent based on the B2M surface. Although findings from this study show progress using advanced senolytics, these results will need to be replicated in mice before being tested on humans.

