New Study Shows Probiotics Slow the Progression of Alzheimer’s
A formulation of 10 human-derived probiotics — beneficial gut bacteria — counter Alzheimer’s progression and improve gut health in mice.
Highlights
- The probiotic consortium, formulated from five Lactobacillus and five Enterococcus species, enhances cognition and reduces brain pathology.
- The formulation also improves gut wall integrity and health, reducing inflammation-triggering gut microorganisms.
A recent study published in Scientific Reports presents compelling evidence that a specific consortium of human-derived probiotic strains may mitigate the progression of Alzheimer’s disease (AD) pathology. This research contributes to the growing body of literature examining the intricate relationship between the gut microbiome — the microorganisms living in our gut that include mostly bacteria — and neurodegenerative disorders.
A Precisely Formulated Probiotic Consortium
The investigators developed a specific probiotic formulation comprising ten bacterial strains isolated from the human infant microbiome. This consortium included five Lactobacillus species (L. paracasei, L. rhamnosus, L. plantarum, L. rhamnosus, and L. plantarum) and five Enterococcus species (E. raffinosus, E. INBio, E. avium, E. avium, and E. avium). These strains were selected based on their previously demonstrated capacity to modulate microbiome composition and reduce inflammatory processes.
How the Probiotic Formula Was Tested
The study employed the APP/PS1 transgenic mouse model, which harbors human amyloid precursor protein (APP) and presenilin-1 (PS1), genetic mutations associated with inherited AD. These animals develop pathology and cognitive deficits that parallel human AD progression. While the control group received standard drinking water, the intervention group received the probiotic formulation (1 × 10^11 CFU daily) in drinking water. The intervention period was extended for 16 weeks, after which comprehensive analyses were conducted.
Probiotics Counter Alzheimer’s Progression
The results demonstrated statistically significant improvements across multiple parameters in the probiotic-treated animals, including enhanced cognitive function, reduced amyloid pathology, attenuated neuroinflammation, and preserved blood-brain barrier (BBB) integrity.
Enhanced Cognitive Function
Cognition was measured by placing mice into a pool of water containing a platform used to escape from drowning. After learning the location of the platform over two test trials, the platform was removed. The time spent in the former location of the platform was then measured during a third trial. The results showed that while control mice often wandered in the wrong location, the probiotic-treated mice immediately swam to the correct location, suggesting improved memory and learning.
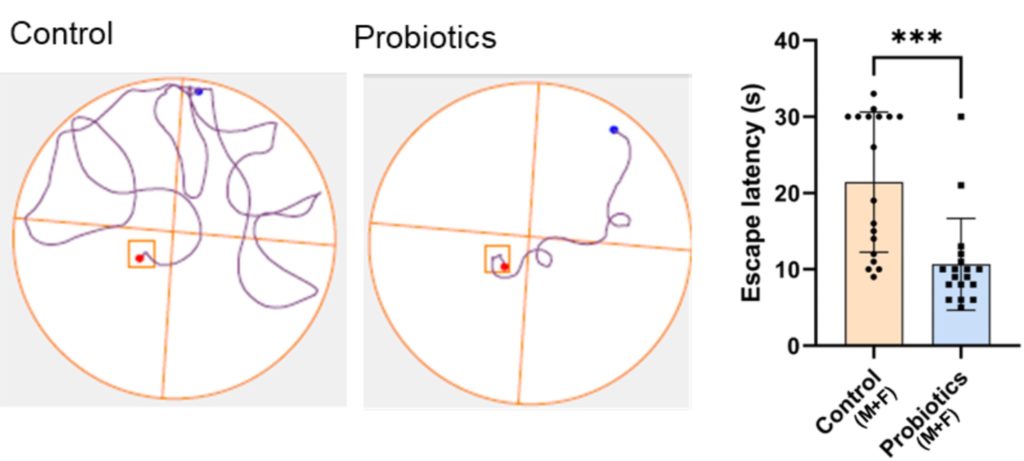
Reduced Brain Pathology
Amyloid-beta is a protein that aggregates in AD brains and is considered a hallmark of AD. In a region of the brain central to consolidating memories, the hippocampus, the researchers found that these proteins were reduced in probiotics-treated mice. The probiotic intervention also reduced inflammatory markers, suggesting modulation of neuroinflammatory processes. Moreover, the BBB, which keeps harmful molecules out of the brain, was maintained in the intervention group, indicating protection against inflammatory disruption.
Role of the Gut Microbiome
The traditional focus of AD research has centered on brain-specific pathologies like amyloid-beta accumulation and neuroinflammation. However, contemporary neuroscience has increasingly recognized influences from outside of the brain. The bidirectional communication between the gastrointestinal system and the central nervous system, termed the gut-brain axis, has emerged as a critical mediator in brain health and disease.
The researchers showed that the probiotic treatment altered microbiome composition, reducing the abundance of pro-inflammatory bacteria that were enriched in the control APP/PS1 mice. Moreover, the intervention maintained intestinal wall integrity, thereby reducing the permeability of inflammatory factors into the bloodstream, as shown by lower blood pro-inflammatory protein levels. Intestinal wall health was also improved, as shown by longer villi, structures that increase the absorption of water and nutrients.
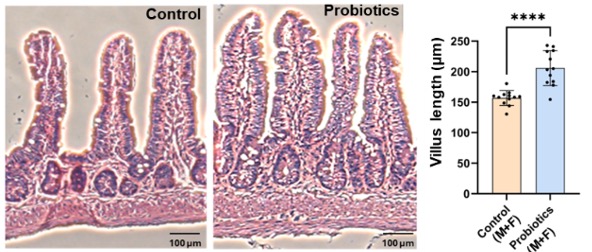
The Big Picture
The data suggest that probiotics reduce intestinal inflammation by altering the composition of the gut microbiome. Without probiotics, intestinal inflammation damages the intestinal wall, allowing for inflammatory proteins, and even gut bacteria, to leak into the bloodstream. The inflammatory proteins travel through the bloodstream to the BBB, causing damage to this important structure. After the BBB has deteriorated, the inflammatory proteins can enter the brain, leading to neuroinflammation and subsequent brain deterioration (neurodegeneration).
By changing the gut microbiome in a way that lessens tissue-deteriorating inflammation, probiotics support intestinal wall integrity. With an intact intestinal wall, inflammation cannot spread to the brain and contribute to neurodegeneration, cognitive dysfunction, and AD.
Human Relevance
While the translation of rodent studies to human therapeutics requires caution, several aspects of this research enhance its potential clinical relevance:
- The probiotic strains utilized were of human origin, suggesting potential compatibility with the human microbiome.
- The intervention was administered non-invasively through drinking water, representing a feasible delivery method for human application.
- The treatment was initiated in young animals before extensive pathology developed, suggesting potential efficacy as an early intervention or preventive approach.
Supporting Human Studies
This study contributes to the expanding field of research examining the microbiome’s influence on brain function. Previous investigations have established several relevant findings:
- Distinctive alterations in microbiome composition have been documented in Alzheimer’s disease patients compared to age-matched controls.
- Fecal microbiota transplantation (FMT) — infusion of healthy fecal matter (containing gut microbiota) into the intestinal tract of an individual with disease — improved mental acuity and memory in an 82-year-old man with a 5-year history of AD.
- Gastrointestinal inflammatory conditions correlate with increased risk for AD.
The current research extends these observations by demonstrating that targeted microbiome modulation can influence Alzheimer’s disease progression, providing further evidence for the gut-brain axis as a therapeutic target.
How to Improve Your Gut Microbiome
While specific probiotic formulations for Alzheimer’s disease prevention remain investigational, this research reinforces the importance of microbiome health in neurological well-being. Current evidence supports several approaches to maintaining microbiome homeostasis:
- Consumption of fiber increases microbial diversity, supporting a healthy gut microbiome, while refined grain and gluten intake reduces microbial diversity.
- Inclusion of naturally fermented foods containing live microbial cultures, such as kimchi, yogurt, sauerkraut, and kefir can improve gut microbiome health.
- Ultra-processed foods may disrupt microbial communities and increase intestinal inflammation and should be avoided. Ultra-processed foods include, Eggo waffles, Minute Maid lemonade, Ritz crackers, Skittles, and Coca-Cola soda.
- Stress management and regular physical activity, both of which influence microbiome composition, may help with improving microbial diversity.
Model: APP/PS1 Alzheimer’s disease model mice
Dosage: 1 × 10^11 CFU of L. paracasei, L. rhamnosus, L. plantarum, L. rhamnosus, L. plantarum, E. raffinosus, E. INBio, E. avium, E. avium, and E. avium formulation per day in drinking water for 16 weeks

