New Study Predicts Maximum Human Lifespan with DNA Chemical Modification Analysis
Spanish researchers unveil data suggesting that higher rates of disorder in chemical modifications to DNA (methylation) are associated with shorter lifespans across 18 mammal species.
Highlights
- Longer-lived mammalian species like humans showed lower rates of disorder in methylation (referred to as epigenetic entropy) than shorter-lived species.
- Statistical analyses of epigenetic entropy data suggested that humans have a maximum lifespan of about 118 years, comparable to the longest-documented human lifespan of 122.5 years.
- For mammals more generally, statistical assessments pointed to a maximum lifespan of 220 years, similar to bowhead whales (the longest-lived mammal) that may live 211 years.
Published in a non-peer reviewed article on bioRxiv, Fraga and colleagues from the University of Oviedo in Spain found that the rate of epigenetic entropy accumulation in different mammalian species is inversely correlated with how long each tends to live. For example, humans and Asian elephants had slower gains in epigenetic entropy, which may help explain why they live for decades. Contrastingly, mice and rats were found to have much faster rates of epigenetic entropy gains, which may aid in explaining their shorter lifespans of a few years. The study could pave the way for people to view aging in a new light, being driven by epigenetic entropy, in a similar manner proposed in Harvard professor David Sinclair’s Information Theory of Aging.
Species with Slower Epigenetic Entropy Gains Live Longer
The Spanish researchers who conducted the study utilized blood samples of 18 different mammalian species. They measured rates of epigenetic entropy accumulation in cells from the blood samples with a technique called Horvath’s mammalian array, which measures DNA methylation patterns across different species of mammals. Thus, with Horvath’s mammalian array, Fraga and colleagues detected an inverse correlation between the rate of accumulation of epigenetic entropy and lifespan. In other words, the research team associated lower rates of epigenetic entropy accumulation with longer lifespans and higher rates with shorter lifespans.
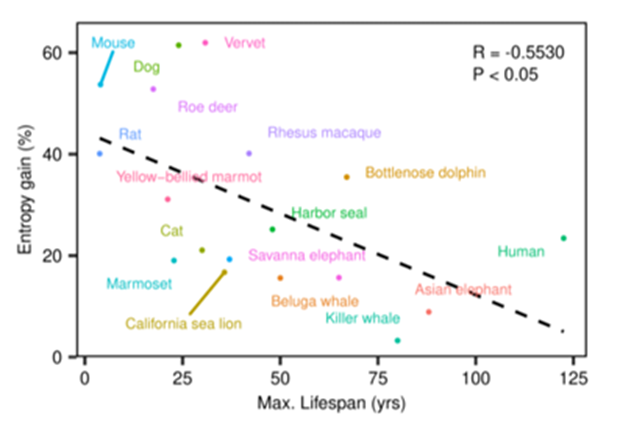
Moreover, based on their statistical assessments of epigenetic entropy data, human lifespan was predicted to max out at around 118 years. This aligns closely with the longest-documented human lifespan of 122.5 years.
As for mammals more generally, Fraga and colleagues’ data hinted that the longest lifespan for any mammal would be around 220 years. In that regard, the longest-lived mammalian species, the bowhead whale, lives around 211 years, nearly matching this prediction.
In general, the statistical predictions of maximum lifespan closely align with those observed in real life. These findings lend statistical credulity to the methods that the Spanish researchers used to inversely correlate epigenetic entropy gains with lifespan and suggest that they may have some real-world application. If that is the case, a key driver of aging very well may be the accumulation of epigenetic entropy over time.
The Possibility of Reversing Epigenetic Entropy with Cellular Reprogramming
This analysis from Fraga and colleagues begs the question of whether methylation patterns seen during an organism’s younger years can be restored to thereby reverse epigenetic entropy. If so, the function of various organs, such as the heart, kidneys, and eyes, could possibly be rejuvenated to a younger state.
Along those lines, some research suggests that increasing cellular levels of proteins called Yamanaka factors, through a process called cellular reprogramming, restores more youthful epigenetic information and also rejuvenates certain tissues. In fact, Dr. David Sinclair and his team have used a form of cellular reprogramming (referred to as partial cellular reprogramming using three out of four Yamanaka factors) to recover youthful methylation patterns and restore eye function and vision in aged mice. This research provides some of the first evidence that using Yamanaka factors to restore youthful DNA methylation information may serve to rejuvenate certain tissues and restore their function. If applicable to humans, cellular reprogramming may serve as a way to slow or reverse epigenetic entropy, rejuvenate tissues, and extend lifespan in some individuals.
All the more, injectable gene therapies utilizing cellular reprogramming were used to restore vision in aged mice. Moreover, Dr. Sinclair and his team have also conducted research on oral drugs that increase cellular levels of Yamanaka factors to reprogram cells to a younger state, which is less invasive than the gene therapy. Thus, once their safety and efficacy have been proven, the possibility of using less invasive oral cellular reprogramming drugs may offer some hope to slow or reverse epigenetic entropy.
If, in fact, epigenetic entropy serves as a primary driver of aging, using gene therapy or newly developed drugs to slow or reverse it may restore the function of tissues throughout the body to help us extend our lives. Perhaps someone living longer than 122.5 years, the longest-recorded human lifespan, will result from an aging intervention based on slowing or reversing epigenetic entropy through techniques like cellular reprogramming.

