New Sirtuin Activator Against Age-Related Diseases Hitting Clinical Trials
A new sirtuin 3 activator with stronger effects than NAD+ precursor NMN will enter clinical trials in 2025, targeting Alzheimer’s and other age-related diseases.
Highlights
- Scientists have engineered molecules capable of nearly doubling the activity of sirtuin 3, a longevity enzyme once thought undruggable.
- One of the new sirtuin 3 activators is more effective than NMN and may help bring mitochondrial health to youthful states.
- Clinical trials for the new sirtuin activators are set to begin in 2025.
Without enzymes, we would not exist. Some biochemical reactions necessary for life would take up to 2.3 billion years without enzymes, which can catalyze the same reaction in milliseconds. However, scientists have recently discovered that vital enzymes, needed to keep our cells alive, decline with age. Among these are a family of enzymes called sirtuins, associated with alleviating age-related conditions such as cardiovascular disease, cancer, type 2 diabetes, and neurodegeneration.
Because of their paramount importance in healthy aging and longevity, sirtuin activators have attracted considerable interest in the field of aging biology and drug development for age-related diseases. While NAD+ (nicotinamide adenine dinucleotide) is known to activate sirtuins via NMN (nicotinamide mononucleotide) and other NAD+ precursors, Guan and colleagues have engineered a molecule with stronger sirtuin activity than NMN.
Engineering a Sirtuin Activator
Our ability to manipulate enzyme activity using small molecules is largely based on the exploitation of enzyme binding sites called allosteric sites. When the right molecule binds to an allosteric site, it changes the shape of that enzyme, either increasing or decreasing its activity. Allosteric sites have evolved over millions of years, so synthesizing drugs to bind these sites is like hacking our biology. However, some enzymes lack known allosteric binding sites, including sirtuin 3 (SIRT3).
SIRT3 is a major mitochondrial sirtuin shown to mitigate mitochondrial dysfunction, a primary driver of aging. Mitochondrial dysfunction exacerbates other drivers of aging, including oxidative stress, which damages proteins, fats, and DNA. Since mitochondria are the main source of cellular energy, their dysfunction also leads to energy deficits. Deficits in cellular energy — ATP — are associated with neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease.
With this in mind, Guan and colleagues sought to engineer a molecule that activates SIRT3 without exploiting an allosteric binding site. To do so, they utilized a known SIRT3 activator called honokiol, a compound from tree bark with therapeutic potential for anxiety, pain, and cognitive disorders like Alzheimer’s disease. While honokiol activates SIRT3 briefly, it does not maintain SIRT3 activation for extended periods, which is needed to counteract age-related SIRT3 decline.

The researchers screened 1.2 million compounds for their SIRT3 activation potential. The screen, which was conducted using computer software, identified the top potential SIRT3 activators, which were then validated in test tubes (in vitro). Ultimately, the test tube experiments revealed that two compounds, numbered 5329973 and 5689785, nearly doubled the activation of SIRT3.
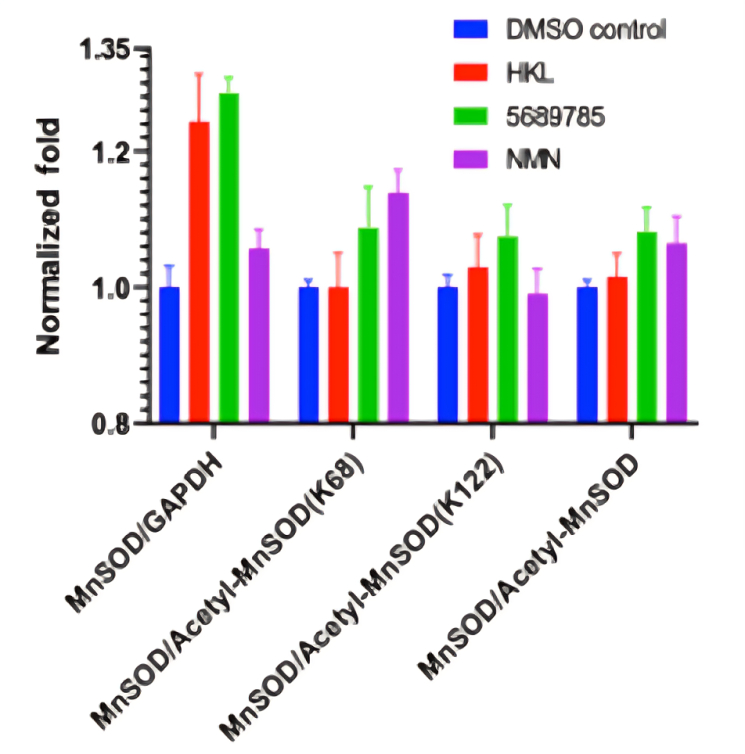
The function of SIRT3 is to remove groups of atoms called acetyl groups (2 carbons, 3 hydrogens, and 1 oxygen) from proteins. One of the more important proteins from which SIRT3 removes acetyl groups is MnSOD (manganese superoxide dismutase). MnSOD is a powerful antioxidant enzyme that counteracts oxidative stress. Compounds 5329973 and 5689785 essentially removed acetyl groups from MnSOD more efficiently than all other compounds screened.
New Sirtuin Activator Stronger than NMN
To compare the effects of their newly discovered SIRT3 activator against existing SIRT3 activators, Guan and colleagues compared compound 5689785 to honokiol and NMN. Overall, compound 5689785 removed acetyl groups from MnSOD more efficiently than honokiol and NMN, suggesting it is the strongest SIRT3 activator of the three. However, SIRT3 removes acetyl groups from multiple lysine amino acids on the MnSOD protein. As such, NMN showed stronger efficacy than honokiol and compound 5689785 with one of the lysines (K68) measured.
New Sirtuin Activators May Halt Cellular Aging
As we age, our NAD+ levels can drop by up to 50%. Moreover, this decline in NAD+ directly affects the activation of SIRT3, which uses NAD+ as a fuel. Guan and colleagues’ new sirtuin activators were shown to nearly double SIRT3 activity in an experimental setup with half the normal mitochondrial NAD+ concentration. This suggests that these activators can normalize SIRT3 activation to youthful levels, potentially rejuvenating mitochondrial health. By restoring mitochondrial health, oxidative stress would be reduced, and ATP production would be revitalized.
Upcoming Clinical Trial
Interestingly, according to SciTechDaily, Chakrabarti Capital Management (CCM) Biosciences will enter “clinical trials in 2025 to target Alzheimer’s, Parkinson’s, and other age-related diseases.” However, this date cannot be found on the CCM Biosciences website and the trial cannot be found on ClincalTrials.gov. Additionally, according to SciTechDaily, “the proposed compounds are also undergoing animal testing in mice for age-related disorders, including infertility, where they have outperformed both NAD+ supplements and other sirtuin activators.” The proposed compounds are more than likely compounds 5329973 and 5689785.
“Efforts have been underway for decades to activate signaling pathways regulated by sirtuins to combat age-related disorders, but prior efforts have encountered significant hurdles. The discoveries by CCM Biosciences pertaining to the design of drug candidates that can activate the major mitochondrial pathways regulated by sirtuins, along with the clinical development plan for evaluation of efficacy as well as safety of these drug candidates, revitalize this area of drug development,” said sirtuin expert Dr. Michael Pollak from McGill University.

