New Research Uncovers Link Between Antibody Buildup and Aging
In what may be the discovery of a new hallmark of aging, researchers uncover the buildup of antibodies in tissue regions with a high prevalence of senescence (a dysfunctional state where cells release inflammatory molecules).
Highlights
- Through the analysis of aging-related gene expression patterns, researchers identified senescence-prone regions of tissue—referred to as senescence-sensitive spots (SSSs)—in mice.
- In areas of tissue surrounding SSSs, the research team observed an accumulation of antibodies (specifically, immunoglobulin G [IgG]).
- The researchers also found that IgG builds up in aged tissues from humans and that reducing IgG mitigated signs of aging across various tissues in mice.
Aging takes its toll on the body in various ways, from muscle loss at the macromolecular scale to inflammation at the cellular level. By focusing on several of these changes, scientists have identified hallmarks of aging.
To receive a designation as a hallmark of aging, these age-related attributes must meet three criteria: they must show age-related manifestation, their experimental elevation should accelerate aging, and therapeutics used against them must decelerate aging. Along those lines, the quest to identify new hallmarks of aging with these criteria never stops since pinpointing them may aid in developing new therapeutics to slow aging.
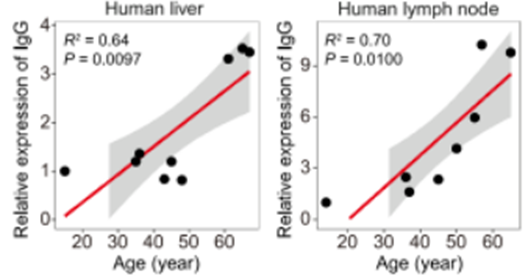
Now, published in Cell, Liu and colleagues from the Chinese Academy of Sciences in Beijing, China provide evidence for perhaps another hallmark of aging—the buildup of IgG in tissues. To uncover this possible new hallmark of aging, the China-based team measured aging-associated gene expression across various tissue types at multiple stages of life in mice. In these tissues, genes with altered expression patterns during aging that overlapped with increased senescent gene activity were used to identify SSSs. Further gene analyses of these SSSs showed higher gene expression for IgG antibodies in the vicinity of SSSs. Building on this evidence, the researchers also found that IgG concentrations increase in aged tissues from mice and humans and that reducing IgG levels alleviated signs of aging in various tissues in mice. Findings from this study could spur research toward finding ways to lower IgG levels in an attempt to slow aging.
IgG is a crucial antibody that helps the immune system fight infections, helping to neutralize toxins, viruses, and bacteria. It is also the most common type of antibody found in human circulation, making up 10% to 20% of blood plasma proteins. However, as suggested by the findings from Liu and colleagues, IgG’s excessive buildup in tissues may drive cellular senescence and inflammation, which are key contributors to aging.
Senescence-Sensitive Spots Overlap with IgG Accumulation
To get a more comprehensive picture of cellular mechanisms that may contribute to aging, Liu and colleagues turned to a technique they called Gerontological Geography. With this method, they compared the expression patterns of aging-related genes between young and old male mice across nine different tissues—the hippocampus, spinal cord, heart, liver, lungs, small intestine, lymph node, spleen, and testis. The researchers found that genes with altered expression levels in these tissues with age were related to chronic inflammation, dysfunction of the cell’s powerhouse (mitochondria), and disrupted nutrient sensing.
Liu and colleagues then looked at tissue regions harboring hotspots for senescence since cellular senescence has been shown to drive inflammation, possibly leading to dysfunctional mitochondria and disrupted nutrient sensing. Through their analysis of five senescence-related genes, Liu and colleagues pinpointed regions where senescence gene expression overlapped with altered expression of other age-related genes in aged mice. The researchers devised an aging score for these regions based on the intersection of senescence genes and genes with altered expression during aging to identify senescence-prone SSSs. Thus, delineating SSSs helped Liu and colleagues to take a closer look at how aging evolves in tissue regions susceptible to senescence.
To then identify culprit genes likely contributing to these SSSs, Liu and colleagues sought to find which age-related genes were the most altered in the SSSs. They found that IgG-associated genes were highly expressed in the vicinity of SSSs. These results show that consistently, across multiple tissues, IgG-associated genes are activated.
To then find whether IgG accumulation may serve as an age-related manifestation, Liu and colleagues measured IgG levels in mice at different stages of aging. They found that, as expected, IgG accumulated with age across organs, yet aging intervention strategies like long-term exercise mitigated IgG buildup. Liu and colleagues also observed a buildup of IgG in human tissue taken from surgery patients and deceased individuals. These findings suggest an accumulation of IgG during aging across multiple stages of life and species for both sexes, suggesting it may serve as a widespread, age-related phenomenon.
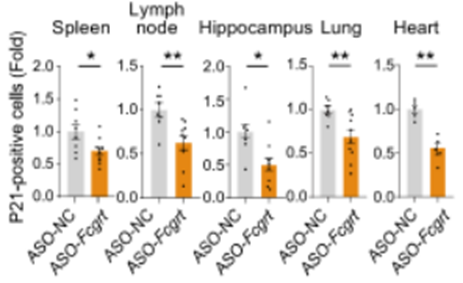
Finally, to find whether reducing IgG levels has an effect on aging, Liu and colleagues used short sequences of DNA and RNA called antisense oligonucleotides to reduce tissue IgG levels in aged mice. After using this technique to lower IgG levels, the researchers found reduced markers of senescence in the spleen, lymph nodes, hippocampus, lungs, and heart. Since senescent cells have been shown to contribute to aging, lowering senescence markers by reducing IgG levels reinforces the notion that accumulated IgG plays a pivotal role in tissue aging. Furthermore, these results also suggest that finding ways to reduce IgG levels may serve to ameliorate aging.
Confirming Elevated IgG as a Hallmark of Aging
The findings from Liu and colleagues provide evidence that IgG buildup during aging may play a critical role in tissue aging progression. In that sense, IgG buildup may eventually serve as a newly-identified hallmark of aging.
As far as potential future human applications go, the data showing that IgG reduction lowers tissue senescence suggests that finding ways to lower IgG antibody buildup may one day thwart the ravages of aging. What’s more, some techniques like immunoglobulin replacement therapy, given to patients with antibody deficiencies, are used to increase IgG levels, so clinicians should be cautious not to overuse them since excessive IgG antibodies may contribute to aging.
“The discovery of IgG as a biomarker points to a potential intervention target. In the future, we might be able to reduce IgG levels related to aging and inflammation in humans,” Gu Ying, a corresponding author, told South China Morning Post.
Model: C57BL/6J mice
Dosage: 10 nmol antisense oligonucleotide-Fcgrt injected intraperitoneally every four days for 10 times total

