New Research Reveals Sleep Medication Could Make Your Brain Age Faster
The removal of metabolic waste from the brain is impaired by the pharmaceutical sleep-aid drug zolpidem in mice.
Highlights:
- For the first time, researchers were able to study the brain physiology of mice during natural sleep.
- Zolpidem, an FDA-approved drug that goes under brand names like Ambien, Edluar, and Zolpimist, disrupts natural sleep physiology, potentially increasing the risk of neurodegeneration.
The number of hours we lie in bed unconscious may not be the best barometer of sleep quality. While we sleep, our brain’s electrical activity traverses several stages, including the rapid eye movement (REM) stage. However, 75% of our total sleep time is spent in the non-REM (NREM) stages, during which our memories are consolidated.
Now, researchers from the University of Copenhagen in Denmark have shown that the brain clears out metabolic waste during NREM sleep. Moreover, the anti-insomnia drug zolpidem blocks this clearance of metabolic waste. These findings may explain why zolpidem is associated with an increased risk for Alzheimer’s disease and neurological disorders.
Brain Physiology During Natural Sleep
Until now, technical barriers have required mice to be anesthetized to model sleeping. However, anesthesia disrupts important features of natural sleep, including the release of norepinephrine — a hormone that constricts blood vessels and reduces blood flow. For this reason, the Danish researchers devised a way to study the brain physiology of naturally sleeping mice using fluorescent sensors.
With their new technique, the researchers showed the intricate connection between the brain’s electrical activity, norepinephrine release, blood flow, and cerebral spinal fluid (CSF) flow. They found that peak impulses in slow-wave brain activity during NREM sleep cyclically released norepinephrine, reducing blood volume and elevating CSF in the brain in waves. Importantly, the researchers showed that waste removal from the brain was correlated with these cyclic physiological changes.
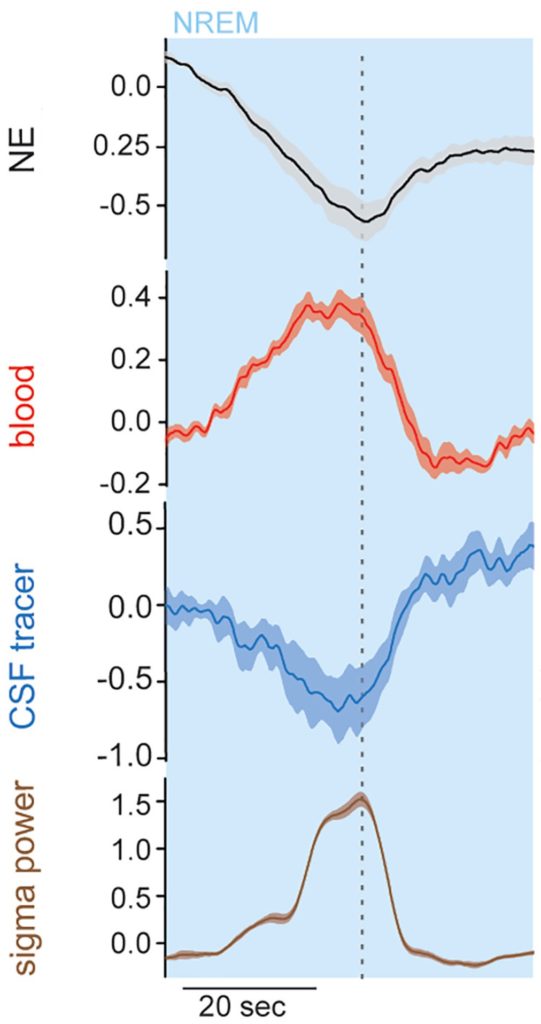
Overall, these findings suggest that slow-wave brain activity during NREM sleep determines the cyclic release of norepinephrine, leading to the cyclic constriction and dilation of the brain’s blood vessels that act like a pump to remove metabolic waste and allow fresh CSF to enter the brain.
Zolpidem Disrupts Natural Brain Physiology
To determine the effect of zolpidem on brain physiology during natural sleep, the researchers injected 5 mg/kg of zolpidem into mice. This led to a decrease in the cyclic release of norepinephrine and a reduction in slow-wave brain activity. Moreover, by placing a tracer into the mice’s CSF, it was found that CSF flow into the brain was reduced in mice given zolpidem, suggesting a reduction in metabolic waste clearance from the brain.
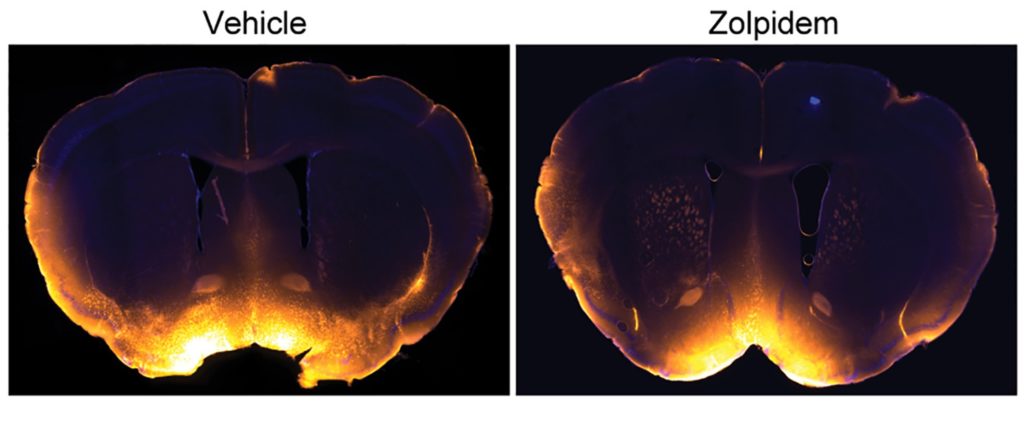
Does Zolpidem Accelerate Brain Aging in Humans?
This study reveals that the pulsation of blood in our brain, mediated by norepinephrine, contributes to the removal of metabolic waste via the CSF during NREM sleep. This means that high blood pressure, linked to stiffened arteries and disrupted constriction and relaxation of the blood vessels, could impair the brain’s waste clearance system. Moreover, blood flow impairments (i.e., impaired hyperemia) are considered a hallmark of aging and neurodegenerative diseases.
While this study provides the conceptual basis for how zolpidem can lead to accelerated brain aging (i.e., neurodegenerative diseases), more studies are needed to ensure that this occurs in humans. Still, studies have shown that zolpidem usage is associated with double the risk of early death, suggesting that zolpidem is not conducive to longevity, perhaps by accelerating brain aging.

