New Study Explains How Ozempic Could Extend Life
Clusters of brain cells involved in feeding behavior are the most vulnerable to aging, providing insights into how caloric restriction — consuming fewer calories — may prolong lifespan.
Highlights:
- The brain cells most vulnerable to aging are located in a structure called the hypothalamus and many surround the third ventricle, which stores spinal fluid.
- The weight loss drug Ozempic can target some of the hypothalamus cells vulnerable to aging, which could lower brain inflammation.
- Caloric restriction may prolong lifespan by protecting against the brain cells most vulnerable to aging.
Nature recently published an in-depth and comprehensive study exploring the effects of aging on single cells from critical brain regions. The results showed that the most affected cells were those involved in appetite, located in the hypothalamus, a brain region associated with modulating the aging of the entire body. These results provide key insights into how regulating our appetite can affect how long we live.
The Neurons Most Vulnerable to Aging
Recent technological advances have allowed scientists to measure the gene expression profiles of single cells from whole brain regions using an approach called single-cell RNA sequencing. Considering that the brain is composed of thousands of cell types, this technology can help uncover which types of cells are most affected by aging.
With this in mind, researchers from the Allen Institute for Brain Science in Seattle, Washington analyzed the gene expression profiles of brain regions from young adult and aged mice. The young adult mice were 2 months old, roughly equivalent to 18 human years, and the aged mice were 18 months old, approximately equivalent to 56 human years.
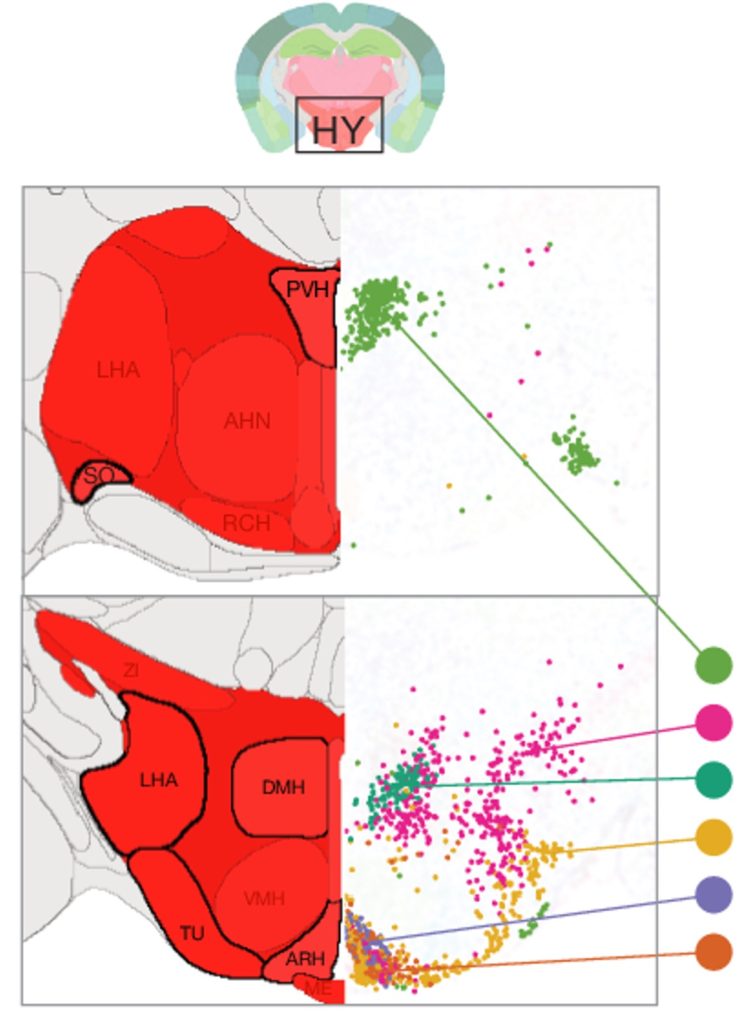
The researchers chose 16 brain regions based on their known sensitivity to aging and age-related diseases. The brain regions covered 35% of all gray matter — the part of the brain containing the bodies of neurons (as opposed to the axons of neurons). Ultimately, over 1 million brain cells, including neurons, were isolated from the mice and 2,449 genes were found to be altered with aging.
The neurons most affected by aging, as measured by the greatest differences in gene expression, were in the hypothalamus. Specifically, the genes that play a critical role in energy balance and satiety. If we intake too much energy, in the form of food, we can become obese. The hypothalamus plays a key role in preventing us from overeating by making us feel full. However, these findings suggest that the neurons involved in appetite become dysregulated with aging.
Neurons Are the Minority
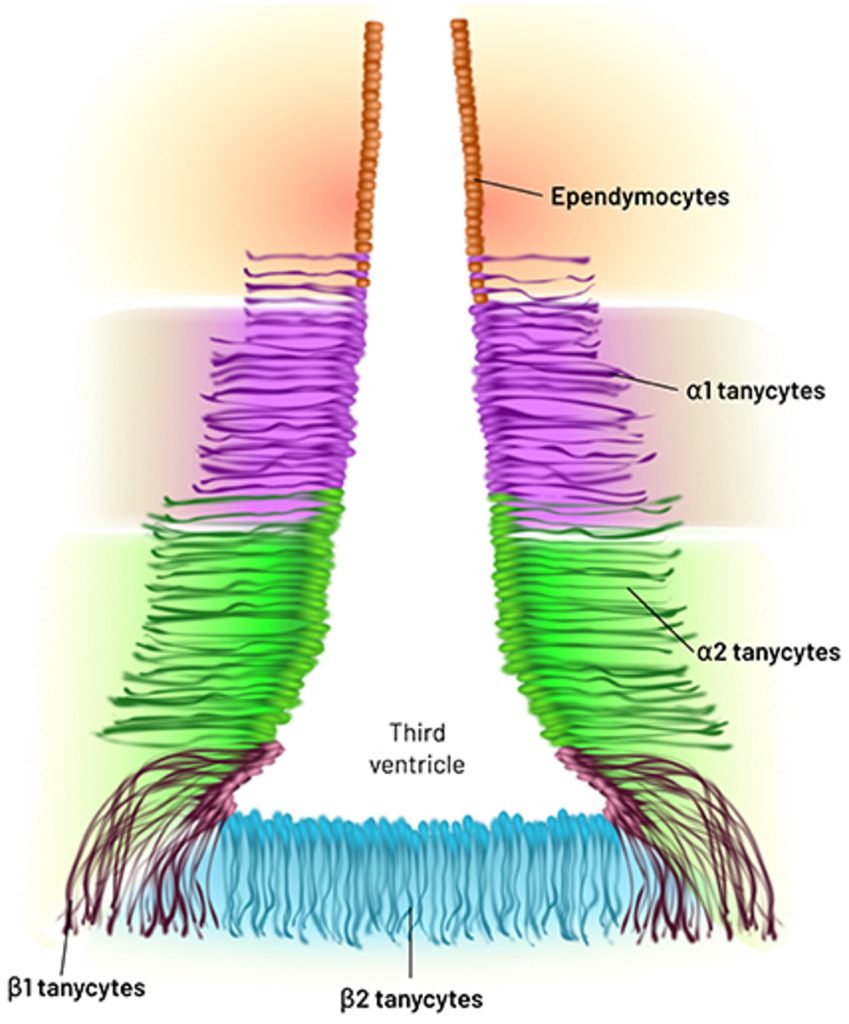
The majority of the cells in our brain are not neurons — the cells that transmit electrical signals — but glia cells, which primarily function to support and protect neurons. Along those lines, the researchers found that the cells most vulnerable to aging were not neurons but glia cells called ependymocytes and tanycytes, both of which line the third ventricle — a cavity in the brain that stores cerebrospinal fluid (CSF) and of which the hypothalamus borders.
Ependymocytes help circulate CSF and provide a barrier between our CSF and bloodstream. Damage to ependymal cells can disrupt the CSF-blood barrier, contributing to neurodegenerative diseases. Tanycytes are exclusive to the lining of the third ventricle in the hypothalamus and contribute to forming the blood-brain barrier and have many functions, including the regulation of food intake.
How Does Ozempic Come into the Picture?
Ozempic is a semaglutide-based diabetes drug, which can effectively reduce the body weight of obese individuals. Ozempic mimics GLP1 (glucagon-like peptide 1), a naturally occurring peptide hormone that suppresses appetite. Both GLP1 and semaglutide bind to GLP1 receptors in the hypothalamus, contributing to our perception of not being hungry.
The researchers found that some of the neurons in the hypothalamus most affected by aging were those expressing GLP1 receptors. Moreover, these GLP1 receptor-expressing neurons also expressed MHC-1 proteins, which signal the immune system to attack and eliminate cells. These results point to an interaction between satiety and inflammation, an underlying biological driver of aging.
Furthermore, considering that GLP1 has been shown to have anti-inflammatory effects, drugs like Ozempic could mitigate brain aging by reducing inflammation. Indeed, semaglutide was associated with a 40% to 70% reduced risk of Alzheimer’s in diabetes patients. However, more studies are needed to determine if semaglutide drugs like Ozempic can reduce brain inflammation and lower the risk of Alzheimer’s in healthy individuals.
How Does Caloric Restriction Prolong Lifespan?
Caloric restriction (CR) — consuming fewer calories than would be eaten freely — has proven to be the most robust intervention to prolong the lifespan of mice. As such, many researchers have chased after discovering compounds that mimic the effects of CR and delay aging. These compounds target the underlying biological drivers of aging called the hallmarks of aging, which include inflammation.
CR also increases NAD+ (nicotinamide adenine dinucleotide), which declines with age and contributes to age-related diseases. Interestingly, stimulating the hypothalamus of mice increases the expression of an enzyme called eNAMPT (extracellular nicotinamide phosphoribosyltransferase), which synthesizes the NAD+ precursor NMN (nicotinamide mononucleotide), which is then made into NAD+. In turn, the stimulation of the hypothalamus and upregulation of eNAMPT prolonged the lifespan of the mice.
Furthermore, evidence suggests that CR targets the cells found to be most vulnerable to aging in this study, including neurons in the hypothalamus that express neuropeptide Y, proopiomelanocortin, and agouti-related peptide. These findings point to CR having a protective effect on these cells, potentially restoring deficits in energy balance and appetite regulation. More studies are needed to determine the role of NAD+ in this process.
While the age-defying effects of CR are still being elucidated, the opposite of CR — overeating — coupled with a sedentary lifestyle has obvious effects, namely obesity. Obesity is known to accelerate the aging process and increase the risk of mortality, cardiovascular disease, cancer, diabetes, arthritis, liver disease, kidney disease, sleep apnea, and depression.
A properly functioning hypothalamus plays a critical role in modulating obesity, as physical damage to the hypothalamus causes hypothalamic obesity — abnormal weight gains due to physical destruction of the hypothalamus. Along those lines, a gradual deterioration of the hypothalamus appears to occur with aging, generating a similar effect to obesity, including inflammation and an increased risk of mortality and chronic diseases.
Overall, behavioral interventions like CR and exercise could potentially protect the hypothalamus from degenerating. Because the hypothalamus modulates the entire body in vital ways, from stress to appetite regulation, its deterioration may contribute to the deterioration of the rest of the body. This could explain how CR prolongs the lifespan of various organisms.

