New NAD World 3.0 Theory Emphasizes NMN’s Importance In Aging
The NAD World 3.0 concept, proposed by Shin-Ichiro Imai, positions NMN as a key player during the process of aging.
Highlights
- In NAD World 3.0, Imai conveys that many researchers have accepted declining cellular nicotinamide adenine dinucleotide (NAD+) levels as a key driving force of aging.
- Recent advances provide evidence for a nicotinamide mononucleotide (NMN) transporter, Slc12a8, and the importance of circulating vesicles containing NMN synthesis enzymes, eNAMPT, that maintain NAD+ levels.
- In the future, it may become possible to unravel how these two NAD+ maintenance elements, Slc12a8 and eNAMPT, function in tissues for targeted pro-longevity therapy.
Since the formal establishment of aging research with the creation of the National Institute on Aging (NIA) in 1974, a clear picture of what exactly aging is and how longevity is determined has not completely emerged. This lack of understanding of aging led Shin-Ichiro Imai of Washington University in St. Louis to describe a parable involving blind men and an elephant in an NPJ Aging publication to illustrate our comprehension of aging.
A Parable to Describe Our Understanding of Aging
The parable is a type of metaphor that cautions against the limitations of human knowledge and experience. In it, three blind men have the task of describing something they are touching, which happens to be an elephant. The three blind men correctly describe how the animal feels yet cannot pinpoint exactly what it is. At the parable’s end, a king reveals to the blind men that what they were trying to describe was a big elephant. Thus, the conundrum that this parable presents is that if there is no king to tell the blind men the truth, then there is no feasible way for the three blind men to perceive that what they were touching is, in fact, an elephant.
In the same vein, our understanding of the underlying pathways and mechanisms underpinning aging has significantly advanced over the past decade. Yet, similar to the blind men trying to describe the elephant, we still do not have a comprehensive picture of what aging is and what determines longevity. Moreover, we also do not have a mighty king to tell us the truth about aging.
The Introduction of the NAD World Concept
The quest to unravel a meaningful understanding of aging and longevity led to the introduction of a concept called “NAD World” in 2009—the first attempt at a comprehensive overview of aging, according to Shin-Ichiro Imai. The NAD World theoretical concept posits a systemic regulatory network, connecting NAD+ metabolism, biological rhythm, aging, and longevity control in mammals.
In this original NAD World concept, two critical elements were proposed to regulate aging and longevity: a cellular enzyme that depends on and consumes NAD+ called SIRT1 and an enzyme involved in NAD+ biosynthesis called NAMPT. In that regard, SIRT1 uses available NAD+ and modulates many physiological processes in cells, such as DNA repair, the sleep-wake cycle (circadian rhythm), metabolism, and aging. Moreover, NAMPT was proposed to generate oscillations of NAD+ production throughout the day in multiple tissues to drive SIRT1 function. In this way, Imai proposed in his original NAD World concept that the coordinated SIRT1 and NAMPT functions control cellular dynamics behind the process of aging and lifespan.
The most important prediction coming from the NAD World concept, according to Imai, was that the driving force behind aging is declining systemic NAD+ levels. Interestingly, some 15 years after this concept’s introduction, the notion that an age-related decline in NAD+ levels works as a driving force behind aging has been accepted as a consensus across the aging research field, according to Imai.
NAD World 2.0
In addition to the original NAD World concept, in 2016, Imai reformulated his theoretical framework and presented a new version called NAD World 2.0. Based on substantial advancements in the aging research field between 2009 and 2016, Imai and colleagues added the involvement of three key tissues to the NAD World 2.0. Accordingly, a brain region called the hypothalamus was identified as a control center for aging that sends signals to ꞵ-adrenoreceptors on fat tissue (adipose tissue) and skeletal muscle. Furthermore, the research team identified skeletal muscle, which utilizes NAD+ for energy generation and contractile force, as a mediator of aging. Adipose tissue, on the other hand, was labeled as a modulator of aging that can generate and secrete NAMPT enzymes contained within vesicles that enter circulation (called eNAMPT), which cells use for NAD+ synthesis.
According to Shin-Ichiro Imai, the most critical prediction of NAD World 2.0 was the secretion of eNAMPT from adipose tissue which facilitates a form of communication between adipose tissue and the hypothalamus. This inter-tissue communication relates to the hypothalamus’s use of eNAMPT for NAD+ biosynthesis.
Another important prediction was the key role of NMN, an NAD+ precursor and product of eNAMPT enzymatic reactions, which modulates the robustness of signaling from the hypothalamus to skeletal muscle and adipose tissue. In that sense, NMN from the NMN-synthesizing enzyme, eNAMPT, maintains NAD+ levels in the hypothalamus. NAD+ level maintenance, in turn, ensures the proper function and signaling of the hypothalamus. Intriguingly, Imai relays that the predictions related to eNAMPT and NMN have both been backed by research performed since NAD World 2.0’s introduction.
Referring to the two critical elements involved in NAD World 2.0, eNAMPT and NMN, Shin-Ichiro Imai integrated his understanding of data acquired since 2016 to reformulate the NAD World 2.0 concept. In doing so, he has presented his latest conception of the NAD World—NAD World 3.0.
NAD World 3.0
NAD World 3.0 combines two key NAD+ level maintenance elements: eNAMPT and a cellular transporter for NMN called Slc12a8. Moreover, NAD World 3.0 has added a fourth tissue, the small intestines, to the NAD World 2.0 model. The inclusion of the small intestines is based on the addition of the NMN transporter, Slc12a8, given that Slc12a8’s localization is primarily in the small intestines. Thus, NAD World 3.0 includes the hypothalamus, skeletal muscle, adipose tissue, and the small intestines.
Evidence Behind Slc12a8
While some researchers have called into question the existence of Slc12a8, multiple studies have given evidence for it. For example, mouse cells with reduced gene expression for Slc12a8 show significantly reduced NMN uptake, whereas restoring gene expression revamps NMN uptake. Furthermore, genetically inducing a deficiency for Slc12a8 in mice’s intestines significantly reduced NMN uptake and diminished intestinal NAD+ levels. Finally, genetically increasing Slc12a8 in the intestines of young mice significantly boosted their intestinal NAD+ levels.
Slc12a8- and eNAMPT-Based NAD+ Biosynthesis Systems
With the addition of the Slc12a8 transporter to the new NAD World 3.0 concept, Shin-Ichiro Imai has proposed that this cellular transporter for NMN helps meet short-term demands for increased NAD+. In that sense, lowered systemic NAD+ levels have been shown to increase the abundance of Slc12a8 transporters in the intestines, which could then increase cellular NMN uptake and subsequent NAD+ synthesis. Since NMN cellular uptake via Slc12a8 occurs in a matter of minutes, Imai has thus proposed that increasing Slc12a8 works to meet short-term NAD+ demands.
On the other hand, another NAD+ synthesis system, circulating eNAMPT vesicles, works in tandem with the Slc12a8 NMN transporter to meet long-term NAD+ demands. eNAMPT vesicles are secreted primarily from adipose tissue and enter circulation in response to neuronal signaling from the hypothalamus. According to Imai, eNAMPT signaling can increase NAD+ levels in certain tissues, such as the hypothalamus, in a matter of hours to days.
In the NAD World 3.0 model, the hypothalamus also signals to skeletal muscle and adipose tissue via the hormones epinephrine and norepinephrine to ꞵ-adrenoreceptors, and since the hypothalamus requires NAD+ for its proper function, NMN modulates the robustness of the hypothalamic signaling. Along those lines, falling NAD+ levels due to reduced NAD+ synthesis from NMN during aging can result in the degradation of this signaling. Thus, whether through Slc12a8 transport in the intestines or synthesis from eNAMPT, NMN plays a key role in the NAD World 3.0 concept that attempts to describe how we age.
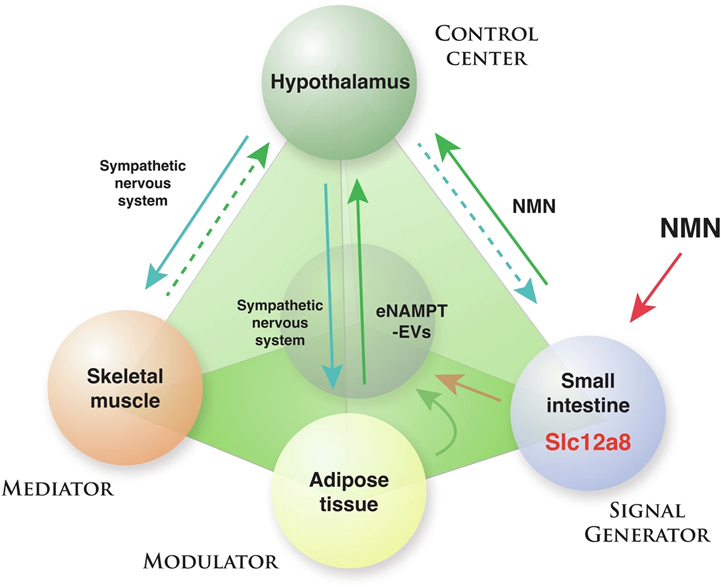
Tissues Vary in Their Use of Either Slc12a8 or eNAMPT for NAD+ Biosynthesis
The short-term or long-term demands for NAD+ synthesis vary by tissue, according to Imai. For example, the liver has Slc12a8 transporters to respond quickly to NMN for NAD+ synthesis. However, the liver does not show significantly increased NAD+ in response to circulating eNAMPT. On the other hand, the hypothalamus shows significantly increased NAD+ levels in response to eNAMPT. Thus, distinct, time-sensitive utilization of these two NAD+ biosynthesis systems, Slc12a8 and eNAMPT, differ across tissues and organs involved in mammalian aging and longevity control. The reasons behind which of the NAD+ biosynthesis elements each tissue uses remain unclear.
Perhaps future research uncovering these mechanisms will aid in rejuvenating certain tissues, such as the hypothalamus, with techniques that increase adipose tissue’s release of eNAMPT. Furthermore, since organs like the liver have Slc12a8 transporters, figuring out how to stimulate these transporters in this tissue in addition to NMN supplementation, may alleviate age-related liver dysfunction.
“Once we fully elucidate how each key inter-tissue communication works, we should be able to start developing effective anti-aging interventions that target and control the main regulatory factors in each feedback loop, such as NMN and eNAMPT-[extracellular vesicles],” said Imai in his NPJ Aging publication.
Shin-Ichiro Imai Proposes Declining Signaling Robustness Within the NAD World 3.0 Model During Aging
Perhaps the most important takeaway from NAD World 3.0 is that it provides potential targets for aging interventions. Along those lines, addressing faulty signaling robustness between the hypothalamus, skeletal muscle, adipose tissue, and intestines, which arises during aging, may serve to prolong life without age-related disease and potentially even lifespan.
Revamping this robustness could entail increasing NAD+ in the hypothalamus. Interestingly, the hypothalamus contains different sets of neurons, some of which use Slc12a8 for NAD+ biosynthesis and others that use eNAMPT. Thus, finding ways to increase eNAMPT and stimulate Slc12a8 transporters in hypothalamic neurons may have aging intervention effects.
Another possibility is the use of NMNH—an NMN molecule with extra electrons. Along those lines, a study showed that while NMN supplementation does not increase NAD+ levels in the brain, supplementation with NMNH does so significantly in mice. In that sense, the possibility looms that increasing NAD+ in the brain’s hypothalamus region with NMNH may serve as a way to restore the robustness of signaling, centered in the body’s proposed control center for aging.
Altogether, the interesting NAD World 3.0 presented by Shin-Ichiro Imai brings NAD+ biochemical reactions within the body as they relate to aging back in the spotlight. Furthermore, researchers, such as Harvard’s David Sinclair, currently have NAD+-related aging interventions undergoing intensive research. With that in mind, only the future will tell what new NAD+- and NMN-related aging interventions currently undergoing research will be made available to the public. If the animal study data suggesting rejuvenating effects from such interventions apply to humans, then effective, clinically-proven NAD+-related aging interventions may become available in the next decade or perhaps sooner.

