Age-Reversal Tech Increases Pregnancy Rates 3-Fold, According to New Study
Reprogramming gene therapy, which may target the root cause of aging, increases the pregnancy rate of middle-aged rats 3-fold, but what does this imply for humans?
Highlights
- Reprogramming gene therapy increases pregnancy rates from 8.3% to 25%.
- Reprogramming gene therapy does not increase the number of pups born, but improves hormone cycles.
- The “age center of the brain” may modulate the onset of infertility by regulating the release of hormones.
In the recent explosion of anti-aging research, an intervention technology capable of potentially reversing aging has emerged — epigenetic reprogramming. Several studies have shown that epigenetic reprogramming prolongs the lifespan of mice. Moreover, reprogramming improves cognition and counteracts Alzheimer’s disease in mice. Now, Gallardo and colleagues show that reprogramming counteracts infertility in rats.
Reprogramming Delays Age-Related Reproductive Decline
To confirm that fertility declines with age, Gallardo and colleagues showed that young rats exhibit a 10-fold higher pregnancy rate than middle-aged rats. While 83% of young rats could get pregnant, only 8.3% of middle-aged rats could, demonstrating an age-related decline in fertility. To determine whether fertility could be improved, the researchers reprogrammed the brain cells of middle-aged rats using gene therapy. As a result, 25% of the reprogrammed middle-aged rats became pregnant, a 3-fold higher pregnancy rate than non-reprogrammed middle-aged rats.
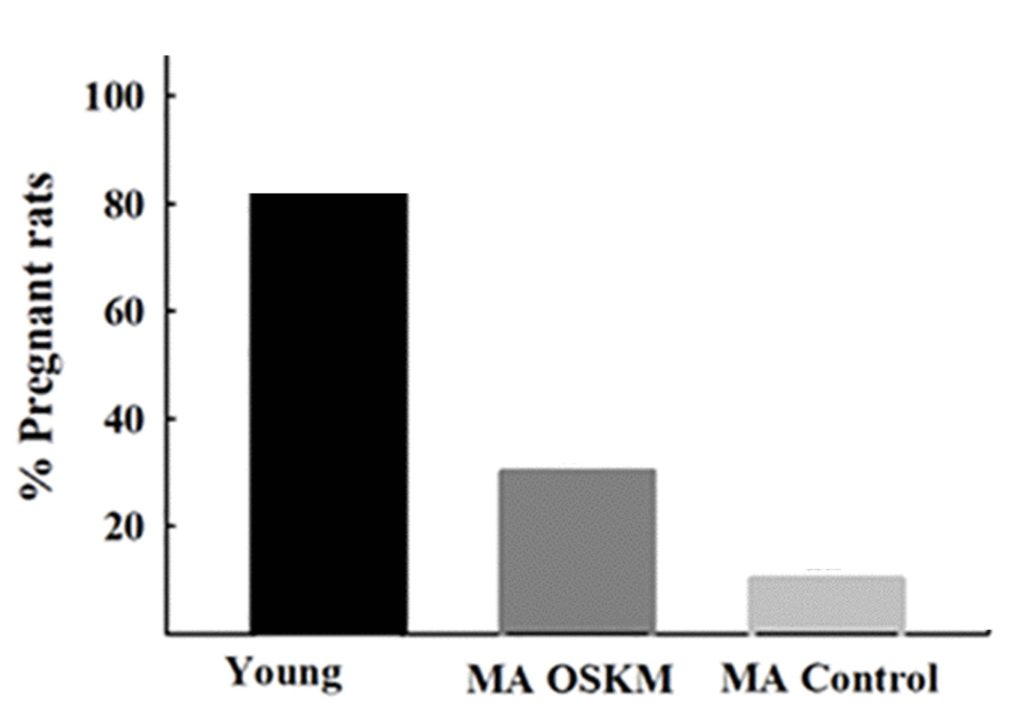
While reprogramming counteracted age-related fertility, it did not affect the number of pups born. The young rats birthed 8 pups, whereas the middle-aged rats, despite being treated with reprogramming therapy or not, had 3 pups. The reprogramming gene therapy did, however, delay changes in estrus cycles, which were irregular in middle-aged rats not given the therapy. These findings suggest that cellular reprogramming gene therapy can counteract and potentially delay female infertility.
What Is Reprogramming?
Reprogramming (partial cellular reprogramming) reprograms our cells to a more youthful state. Because DNA contains the genes for all cell types, some genes must be turned on and some turned off to maintain the identity of each cell. For example, a neuron has neuron-related genes turned on, maintaining its identity as a neuron. A neuron also has heart cell-related genes turned off, because a neuron is not a heart cell.
Epigenetic modifications, modifications that change the three-dimensional structure of DNA, are what determine which genes in a cell are turned on or off. With age, these epigenetic modifications are altered, leading to changes in which genes are turned on or off. According to David Sinclair’s Information Theory of Aging, this causes cells to lose their identity, triggering a cascade of aberrant cellular events that drive biological aging.
With recent advances in molecular biology, researchers can now restore the epigenetic modifications that are altered with age. The technique used to restore the epigenetic landscape of cells is called epigenetic reprogramming. Reprogramming is achieved with four transcription factors called Yamanaka factors, named after Nobel laureate Shinya Yamanaka. The Yamanaka factors are highly expressed in embryonic cells and are used experimentally to revert adult cells into stem cells.
However, when the Yamanaka factors are expressed transiently in aged cells, they restore the epigenetic landscape without causing the cells to revert completely into stem cells. Hence, partial cellular reprogramming reverses the biological age of cells, potentially targeting the root cause of aging.
How Can the Brain Control Fertility?
Menopause, the permanent cessation of menstruation, marks the end of a woman’s reproductive lifespan. Menopause is usually explained by the exhaustion of eggs in the ovaries—ovarian follicular depletion. However, it is now thought that changes to hormones released from the brain precede ovarian depletion. Hormonal changes may even accelerate ovarian depletion, suggesting that restoring normal hormone release could prolong the female reproductive lifespan.
Sometimes referred to as the aging center of the brain, the hypothalamus secretes gonadotropin-releasing hormone (GnRH). It is the release of GnRH that triggers a cascade of events and hormonal changes that characterize the menstrual cycle. Studies have shown that middle-aged women entering menopause have abnormalities in this hormonal cascade. Namely, the surge in luteinizing hormone (LH) that triggers ovulation and fertility. When compared to young women in their early twenties, the LH surge is reduced in middle-aged women.
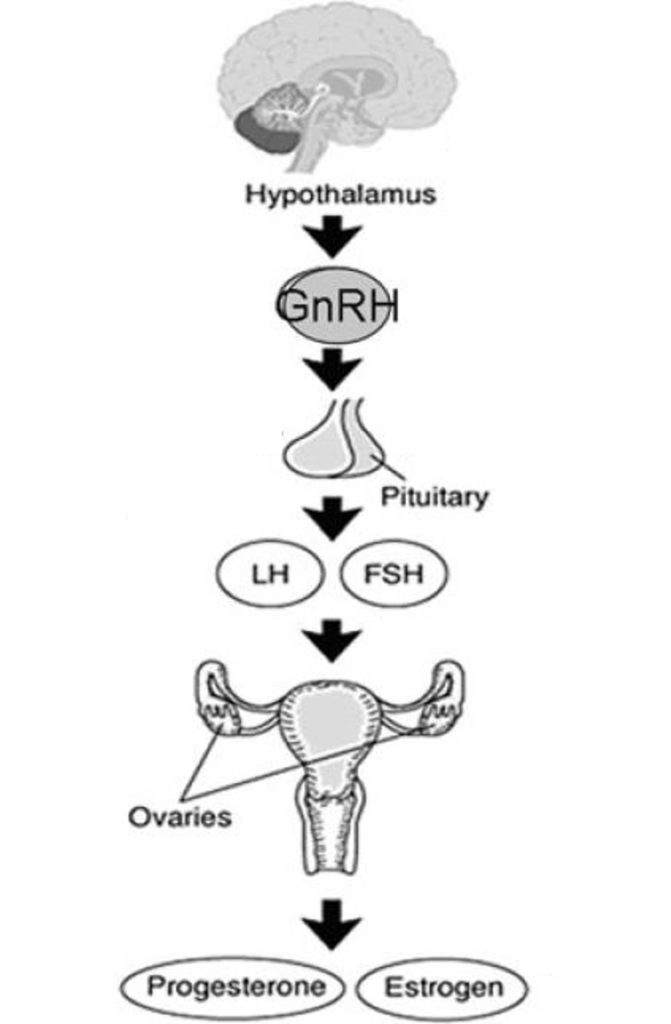
When estrogen is released from the ovaries in response to GnRH, it enters the bloodstream, circulating back to the hypothalamus. Prior to ovulation, estrogen signals the hypothalamus to release more GnRH. This ultimately triggers the surge in LH that begins ovulation.
Rat studies suggest that the attenuation of the LH surge in middle age is due to poor neuronal signaling in the hypothalamus. That is, when estrogen attempts to signal the hypothalamus to release more GnRH, it fails, possibly due to faulty circuitry. Since brain circuitry degenerates with age, the diminished LH surge in middle-aged women may be due to the degeneration of the hypothalamus, whereby the neurons of the hypothalamus are dysfunctional.
It follows that the reprogramming of hypothalamic neurons could improve the brain circuitry needed to restore the release of GnRH in response to estrogen, prompting a robust LH surge. With the LH surge intact, ovulation can be triggered to improve fertility.
The Future of Cellular Reprogramming Gene Therapy
Gene therapy is a technique that uses genes to treat, prevent, or cure disease. While aging is not considered a disease, it is a major risk factor for multiple diseases, including dementia, cancer, and cardiovascular disease. Aging also leads to reproductive senescence in women, which increases the risk of diseases like osteoporosis. Thus, in the future, we may see gene therapy being used to treat, prevent, or cure conditions of aging, including infertility.
For gene therapy to work, genes must be delivered to the inside of cells without being degraded. To achieve this, genes, such as the four Yamanaka genes, can be placed into an empty viral vector (where the virus genes have been removed). A viral vector uses the same mechanism that viruses use to get genes into cells. Gallardo and colleagues utilized the viral vector technique, injecting Yamanaka factor-containing vectors directly into the hypothalamus of middle-aged rats.
However, in humans, it may not be practical to directly inject genes into the hypothalamus because it would require open brain surgery. Instead, scientists need to find how to direct viral vector-delivered genes to specific tissues, like the hypothalamus, after injecting them into the bloodstream. This would be far less invasive and therefore more practical than opening the skull. While some viral vectors are tissue-specific, more studies are needed to refine viral-mediated gene therapy. This refinement not only includes limiting off-target effects but also reducing immune rejection and assuring that genes do not integrate into DNA, which could lead to cancer.
Moreover, we are far from testing cellular reprogramming in humans, as it is an emerging technique. One of the major worries with reprogramming in humans is that it can trigger cancer. Assuming no scientific breakthroughs, it may be decades before reprogramming gene therapy is put into widespread medical use.

