New Findings Reveal Urolithin A Can Help Thwart Alzheimer’s: A Remarkable Discovery
Researchers find that urolithin A enhances learning and memory, mitigates Alzheimer's hallmarks, and protects mitochondria in mice modeling Alzheimer's disease.
Highlights:
- Supplementing mice modeling Alzheimer’s disease with urolithin A significantly enhances cognitive function.
- Long-term UA treatment ameliorates key Alzheimer’s disease pathologies, including reduced amyloid beta plaques and phosphorylated tau levels.
- UA decreases brain inflammation and protects against mitochondrial dysfunction.
In a recent study, scientists at the National Institute on Aging and Tongji University investigated urolithin A as a potential new therapeutic approach to combat Alzheimer’s disease (AD). The study, published in Alzheimer’s & Dementia, reveals that long-term UA treatment significantly enhances cognitive functions and mitigates AD pathology in mice modeling AD. What’s more, the study’s researchers found that UA decreases brain inflammation and protects against mitochondrial dysfunction, a known driver of AD.
The Toll of Alzheimer’s Disease
Afflicting over 6.5 million individuals in the US alone, AD is a progressive neurodegenerative disorder that gradually rids the mind of memories, cognitive faculties, and the ability to perform basic daily functions. Eventually, the disease completely strips away what makes us uniquely human, namely our personality and emotions.
Researchers have yet to pinpoint the root cause of AD. That being said, studies have identified several key hallmarks. One is the buildup of toxic amyloid beta (Aβ) proteins, which triggers inflammation, kills neurons, and impairs synapses – neuronal junctions that allow electrical signals to be transmitted between neurons. Another hallmark is the accumulation of structures called neurofibrillary tangles, which are aggregates of phosphorylated tau proteins that also compromise neuronal communication.
Notably, studies have tied AD progression to mitochondrial dysfunction, which leads to decreased energy production and increased oxidative stress within neurons. This mitochondrial impairment exacerbates the neurodegenerative processes, contributing to the overall decline in brain function observed in AD patients.
What We Know About UA
UA is a natural longevity-linked compound found in foods like pomegranates, berries, and nuts. Although humans naturally produce UA, most individuals do not produce sufficient amounts due to deficiencies in their gut microbiome – an internal hub housing trillions of diverse nutrient-boosting microorganisms. With this in mind, individuals have turned to direct UA supplementation.
Remarkably, UA has been shown to enhance physical performance in overweight adults and delay the onset of muscular aging in mice. Additionally, UA has been found to increase levels of nicotinamide adenine dinucleotide (NAD+), an essential molecule that enhances energy production, DNA repair, and metabolism. More importantly, UA stimulates mitophagy – the clearance of defective/dead mitochondria. Collectively, the literature suggests that UA has the potential to protect against AD.
UA Enhances Memory and Cognitive Abilities
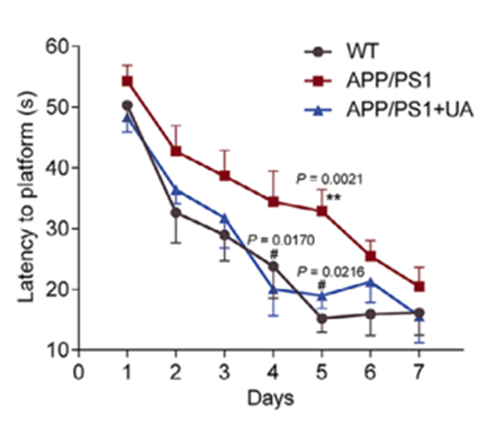
In light of UA’s neuroprotective potential, the study’s investigators performed several behavioral tests commonly used to assess learning and memory. Using mice modeling AD, the investigators supplemented 3-month-old mice with UA for six months and conducted the following tests following treatment: Morris water maze test, Y-maze test, and the novel object recognition test.
Strikingly, UA-treated mice exhibited significant improvements in spatial learning and memory in the Morris water maze, better working memory in the Y maze, and enhanced recognition memory in the novel object recognition test. Notably, even after stopping treatment, the cognitive benefits persisted, demonstrating the compound’s lasting impact. Taken together, the findings suggest that UA could be a powerful therapeutic agent that helps stave off cognitive decline associated with AD.
UA Thwarts AD Hallmarks
Beyond behavioral improvements, UA treatment ameliorated the primary hallmarks of AD. In fact, treating AD mice with UA led to a significant reduction in Aβ plaques in the prefrontal cortex – a brain region hit hard by AD that is responsible for complex cognitive behaviors, decision-making, personality expression, and moderating social behavior. Moreover, tests revealed a notable decrease in phosphorylated tau levels, further indicating UA’s effectiveness at mitigating AD hallmarks.
The investigators also measured markers of inflammation, another known driver of aging and neurodegeneration. Levels of key inflammatory molecules, such as IL-1B and TNF-a, were markedly decreased after long-term UA supplementation. Furthermore, UA treatment reduced the activity of microglia and astrocytes, which are brain cells involved in the inflammatory response. So by quenching inflammation and calming these cells, UA creates a more supportive environment for brain cell health and function.
Previous research in worms found that boosting mitophagy with UA ameliorates AD hallmarks and cognitive decline. For this reason, the investigators confirmed whether long-term UA supplementation stimulates mitophagy in AD mice. They found that long-term UA treatment significantly increased the levels of mitophagy-related proteins PINK1 and Parkin, indicating enhanced removal of damaged mitochondria. Accordingly, these findings suggest that UA potentially protects against AD progression via mitophagy.
UA’s Current Impact
The study’s findings are promising, but it is unclear whether they will translate to humans. Therefore, further testing in humans is needed to fully elucidate UA’s effects on AD and longevity. That being said, UA supplementation is becoming more popular by the day. In fact, the mother of Elon Musk endorses Mitopure, a supplement containing UA, as a cutting-edge anti-aging supplement. While her endorsements may seem convincing of UA’s effectiveness, consulting with a primary care physician before starting supplementation is important.
Model: Male APP/PS1 mice and their wild-type (WT) littermates
Dosage: UA (200 mg/kg/day, Molbase) by gavage starting at 2 months and ending at 7 months when behavioral and molecular endpoints were assessed

